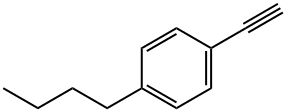
1-Butyl-4-eth-1-ynylbenzene synthesis
- Product Name:1-Butyl-4-eth-1-ynylbenzene
- CAS Number:79887-09-5
- Molecular formula:C12H14
- Molecular Weight:158.24
![Benzene, 1-butyl-4-[2-(trimethylsilyl)ethynyl]-](/CAS/20210305/GIF/202524-78-5.gif)
202524-78-5
1 suppliers
inquiry

79887-09-5
176 suppliers
$10.00/1ml
Yield:79887-09-5 90%
Reaction Conditions:
with methanol;potassium carbonate at 20; for 4 h;
Steps:
Preparation of 1,2-Bis(4-butylphenyl)ethyne (4c).
To a solution of Pd(OAc)2 (13.5 mg, 0.06mmol), PPh3 (31.5 mg, 0.12 mmol), CuI (5.7 mg, 0.03 mmol), and 1-bromo-4-butylbenzene (639 mg,3.00 mmol) in triethylamine (1 mL) was added a solution of trimethylsilylacetylene (324 mg, 3.30mmol). After the mixture was stirred at 80 oC for 4 h, the reaction mixture was acidified with 1NHCl aq. Water and EtOAc were added, and then the organic layer was separated. The aqueous layerwas extracted with EtOAc. The combined organic layer was washed with brine, dried over MgSO4,filtered through a pad of Celite, and then evaporated under reduced pressure. The residual brown oilwas chromatographed on silica gel with hexane/EtOAc = 100/1 (Rf = 0.43) to give1-(4-butylphenyl)-2-trimethylsilylacetylene (S8) as pale-yellow oil (550 mg, 2.40 mmol, 80 %). Theanalytical data were identical to the literature.S15 A suspension of S8 (550 mg, 2.40 mmol) andK2CO3 (495 mg, 3.58 mmol) in MeOH (12 mL) was stirred at room temperature for 4 h. Thereaction mixture was filtered through Celite with EtOAc, and the solvent of the filtrate was removedunder reduced pressure. Water and EtOAc were added, and then the organic layer was separated. Theaqueous layer was extracted with EtOAc. The combined organic layer was washed with brine, driedover MgSO4, filtered through a pad of Celite, and then evaporated under reduced pressure. Theresidual yellow oil was chromatographed on silica gel with hexane/EtOAc = 50/1 (Rf = 0.50) to give1-butyl-4-ethynylbenzene (S9) as pale-yellow oil (340 mg, 2.15 mmol, 90%). The analytical datawere identical to the literature.S16 To a solution of Pd(OAc)2 (8.8 mg, 0.039 mmol), PPh3 (20.5 mg,0.078 mmol), CuI (3.7 mg, 0.020 mmol), and S9 (416 mg, 1.95 mmol) in triethylamine (1 mL) wasadded a solution of 1-butyl-4-ethynylbenzene (340 mg, 2.15 mmol). After the mixture was stirred at80 oC for 4 h, the reaction mixture was acidified with 1N HCl aq. After the same work-up performedin the synthesis of S8, the residue was chromatographed on silica gel with hexane/EtOAc = 50/1 (Rf= 0.30) to give 4c as yellow solid (340 mg, 1.17 mmol, 60 %). The analytical data were identical tothe literature.S17
References:
Kamikawa, Ken;Den, Hiroakira;Tsurusaki, Akihiro;Nagata, Tomoya;Miura, Masahiro [Bulletin of the Chemical Society of Japan,2018,vol. 91,# 7,p. 1069 - 1074] Location in patent:supporting information
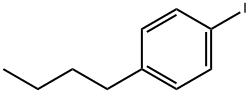
20651-67-6
92 suppliers
$15.00/1g
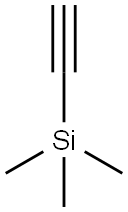
1066-54-2
476 suppliers
$9.00/10g

79887-09-5
176 suppliers
$10.00/1ml
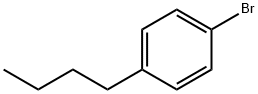
41492-05-1
258 suppliers
$5.00/10g

79887-09-5
176 suppliers
$10.00/1ml

20651-67-6
92 suppliers
$15.00/1g

79887-09-5
176 suppliers
$10.00/1ml

41492-05-1
258 suppliers
$5.00/10g

1066-54-2
476 suppliers
$9.00/10g

79887-09-5
176 suppliers
$10.00/1ml
