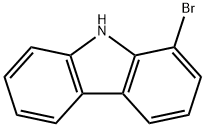
1-Bromo-9H-carbazole synthesis
- Product Name:1-Bromo-9H-carbazole
- CAS Number:16807-11-7
- Molecular formula:C12H8BrN
- Molecular Weight:246.1
Production method of 1-bromocarbazole
86-74-8
336 suppliers
$10.00/5g

16807-11-7
200 suppliers
$6.00/100mg
Yield:16807-11-7 95%
Reaction Conditions:
with N-Bromosuccinimide in toluene at 20; for 0.5 h;Reagent/catalyst;Temperature;Solvent;
Steps:
16; 40; 43 Example 40: Preparation of 1-bromocarbazole (16) from carbazole.
In an air atmosphere, add iron trifluoromethanesulfonate (2mol%, 1.8mg), carbazole (0.2mmol, 33.4mg), NBS (0.22mmol, 39.2mg) and a magnetic stirrer into a 35mL glass pressure tube middle. Then toluene (10 mL) was added, and the reaction tube was reacted at room temperature for 30 min. After the reaction was completed, 10 mL of water was added, extracted with 30 mL of dichloromethane, the organic phase was collected, and the organic phase was dried with anhydrous sodium sulfate. The organic layer was analyzed by gas chromatography, and the yield of the target product was calculated to be 95%.
References:
CN113620811,2021,A Location in patent:Paragraph 0091-0094; 0186-0188; 0195-0197
27372-41-4
1 suppliers
inquiry

16807-11-7
200 suppliers
$6.00/100mg
![1H-Isoindole-1,3(2H)-dione, 2-[(2-bromophenyl)phenylamino]-](/CAS/20210305/GIF/262606-95-1.gif)
262606-95-1
0 suppliers
inquiry

16807-11-7
200 suppliers
$6.00/100mg

86-74-8
336 suppliers
$10.00/5g

16807-11-7
200 suppliers
$6.00/100mg
1592-95-6
397 suppliers
$5.00/1g
67242-17-5
37 suppliers
inquiry

16807-11-7
200 suppliers
$6.00/100mg