
1-BOC-6-AMINOINDOLE synthesis
- Product Name:1-BOC-6-AMINOINDOLE
- CAS Number:219508-62-0
- Molecular formula:C13H16N2O2
- Molecular Weight:232.28
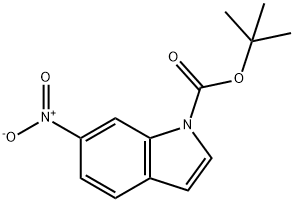
219552-64-4
51 suppliers
$10.00/100mg
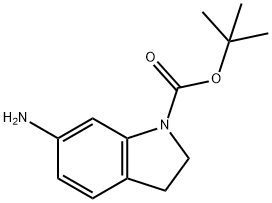
129488-00-2
86 suppliers
$77.00/50 mg

219508-62-0
112 suppliers
$32.00/250mg
Yield:-
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol;ethyl acetate under 2585.81 Torr; for 14 h;Overall yield = 100%; Overall yield = 3.34 g;
Steps:
17.A Part A.
Part A. To tert-butyl-6-nitro-1H-indole-1-carboxylate (3.80 g, 14 mmol), prepared according to Part F of Example 14 using 6-nitro-1H-indole as starting material, was added 150 mL of MeOH and 150 mL of EtOAc. The solution was covered with a stream of N2, and 10 wt % Pd/C (100 mg) was added in one portion. The mixture was subjected to a hydrogen atmosphere (50 psi) for 14 h and was then filtered and concentrated. Analysis (LC/MS) of the resulting brown residue (3.34 g, 100%) showed it to be a 20:80 mixture of tert-butyl 6-amino-1-indolinecarboxylate: tert-butyl-6-amino-1H-indole-1-carboxylate. LC/MS (ESI+): 233.1 (indole M+H)+, 235.1 (indoline M+H)+.
References:
Bristol-Myers Squibb Company;Pinto, Donald J.P.;Quan, Mimi L.;Orwat, Michael J.;Li, Yun-Long;Han, Wei;Qiao, Jennifer X.;Lam, Patrick Y.S.;Koch, Stephanie L. US2017/50964, 2017, A1 Location in patent:Paragraph 0741