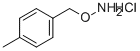
1-[(AMINOOXY)METHYL]-4-METHYLBENZENE HYDROCHLORIDE synthesis
- Product Name:1-[(AMINOOXY)METHYL]-4-METHYLBENZENE HYDROCHLORIDE
- CAS Number:38936-62-8
- Molecular formula:C8H12ClNO
- Molecular Weight:173.64

38936-61-7
3 suppliers
inquiry
![1-[(AMINOOXY)METHYL]-4-METHYLBENZENE HYDROCHLORIDE](/CAS/GIF/38936-62-8.gif)
38936-62-8
25 suppliers
$45.00/50mg
Yield:38936-62-8 69%
Reaction Conditions:
Stage #1: 2-((4-methylbenzyl)oxy)isoindoline-1,3-dionewith hydrazine hydrate monohydrate in tetrahydrofuran at 20; for 1 h;
Stage #2: with hydrogenchloride in 1,4-dioxane at 20; for 0.5 h;
Steps:
2.2 Step 2: O-(4-methylbenzyl)hydroxylamine hydrochloride (XIVb)
To a stirred solution of 48 XIIIb (2.0 g, 7.49 mmol) in 32 THF (20 mL) was added 80% 33 hydrazine hydrate (700 mg, 14.98 mmol) at room temperature and stirred for 1 h. The reaction mixture was filtered, and the filtrate was evaporated under vacuum to give crude 50 XIVb (1 g). The crude product was dissolved in 1,4-dioxane (10 mL), 4N HCl in dioxane (10 mL) was added, and the system was stirred for 30 min at room temperature. The reaction mixture was concentrated and triturated with pentane (10 mL) and diethyl ether (10 mL) to give XIVb (900 mg, 69% yield) as a white solid. LCMS: m/z: 138 [M+H]+ observed, RT=1.78 min (Method B).
References:
US2019/169128,2019,A1 Location in patent:Paragraph 0217; 0219
![O-[(4-Methylphenyl)methyl]hydroxylamine](/CAS/GIF/83670-44-4.gif)
83670-44-4
22 suppliers
$340.00/1g
![1-[(AMINOOXY)METHYL]-4-METHYLBENZENE HYDROCHLORIDE](/CAS/GIF/38936-62-8.gif)
38936-62-8
25 suppliers
$45.00/50mg
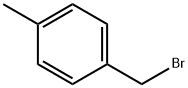
104-81-4
207 suppliers
$17.00/5g
![1-[(AMINOOXY)METHYL]-4-METHYLBENZENE HYDROCHLORIDE](/CAS/GIF/38936-62-8.gif)
38936-62-8
25 suppliers
$45.00/50mg
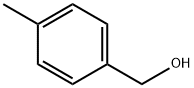
589-18-4
317 suppliers
$5.00/5g
![1-[(AMINOOXY)METHYL]-4-METHYLBENZENE HYDROCHLORIDE](/CAS/GIF/38936-62-8.gif)
38936-62-8
25 suppliers
$45.00/50mg
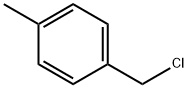
104-82-5
373 suppliers
$10.00/5mL
![1-[(AMINOOXY)METHYL]-4-METHYLBENZENE HYDROCHLORIDE](/CAS/GIF/38936-62-8.gif)
38936-62-8
25 suppliers
$45.00/50mg