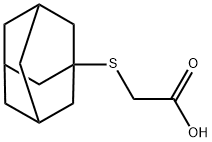
(1-ADAMANTYLSULFANYL)ACETIC ACID synthesis
- Product Name:(1-ADAMANTYLSULFANYL)ACETIC ACID
- CAS Number:95769-28-1
- Molecular formula:C12H18O2S
- Molecular Weight:226.34
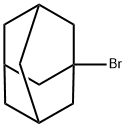
768-90-1
410 suppliers
$10.00/5g
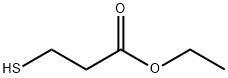
5466-06-8
176 suppliers
$69.00/25g

95769-28-1
14 suppliers
$75.00/100mg
Yield:95769-28-1 24%
Reaction Conditions:
Stage #1: 1-Adamantyl bromide;3-mercaptopropionic acid ethyl esterwith acetic acid for 18 h;Heating / reflux;
Stage #2: with methanol;lithium hydroxide in tetrahydrofuran at 20; for 4 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;methanol; pH=6.5;
Steps:
(Adamantan-l-ylsulfanyl)-acetic acid (XDS04074B); The mixture of 1-bromoadamantane (2 g, 9.35 mmol) and 3-mercapto-propionic acid ethyl ester (2.2 g, 16.4 mmol) in acetic acid (25 mL) was refluxed for 18 h, cooled to room temperature and concentrated in vacuo. The crude oil was partitioned between ethyl acetate and brine. The organic phase was washed with brine, dried over sodium sulphate and concentrated in vacuo to give a residue, which was dissolved in methanol-THF (30 mL - 10 mL). LiOH (550 mg) was added to the solution. After 4 h at ambient temperature, the mixture was neutralized to pH 6.5 with 4N HCl and extracted with ethyl acetate. The organic phase was washed with brine, dried over sodium sulphate and concentrated in vacuo to yield the title compound as white solid (0.5 g, 24 %). mp 71-73 °C; TLC single spot at Rf. 0.29 (30 % ethyl acetate/DCM); 1H NMR (270 MHz, CDCl3) δ 1.62-1.68 (6H, m, 3 x CH2), 1.85 (6H, d, J= 2.2 Hz5, 3 x CH2), 2.05 (3H, broad s, 3 x CH) and 3.30 (2H, s, CH2);-LC/MS (APCI) m/z 227 (M++..).
References:
WO2006/100502,2006,A1 Location in patent:Page/Page column 144