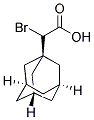
1-ADAMANTYL(BROMO)ACETIC ACID synthesis
- Product Name:1-ADAMANTYL(BROMO)ACETIC ACID
- CAS Number:59768-70-6
- Molecular formula:C12H17BrO2
- Molecular Weight:273.17
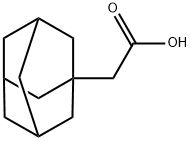
4942-47-6
298 suppliers
$6.00/1g

59768-70-6
19 suppliers
$283.00/100mg
Yield:59768-70-6 82%
Reaction Conditions:
Stage #1: (adamant-1-yl)-acetic acidwith thionyl chloride in DMF (N,N-dimethyl-formamide) at 20; for 1.5 h;
Stage #2: with N-Bromosuccinimide in DMF (N,N-dimethyl-formamide) at 60 - 65; for 3 h;
Stage #3: with water in tetrahydrofuran at 20; for 16 h;
Steps:
17 EXAMPLE 17; Bromination of tricyclo [3.3.1.1 37] decane-1-acetic acid (Formula N) to a-bromotricyclo [3.3.1.1 decane-1-acetic acid (Formula O) Solid tricyclo [3.3.1.1 37] decane-1-acetic acid (Formula N)
Solid tricyclo [3.3. 1. 137] DECANE-1-ACETIC acid (Formula N) (288 grams; 1.48 mole) was suspended in thionyl chloride (465mL) in a 3-necked round bottomed flask, equipped with a condenser. Dimethylformamide (DMF; 0.3 mL was added and the suspension was stirred at room temperature for 1.5 hours. Completion of the reaction was checked by gas chromatography. Solid NBS (307 g) was then added portionwise to the reaction mixture and the reaction mixture was heated to 60C. The reaction was stirred for 3 hours while maintaining the temperature at 60 to 65C. Monitoring by gas chromatography was performed to ensure the completion of the reaction. Heptane (900ML) was added to the reaction mixture. Excess thionyl chloride was distilled off at 78-80C. Water was then added cautiously (violent reaction) to quench the reaction (total volume 1050 mL). Heptane (500 mL) and water (600mL) were then added and the aqueous layer was separated from the organic layer. The organic layer was washed with additional water (600 mL) and the aqueous layer was again separated from the organic layer. Additional water (150 mL) was added to the organic heptane layer and the heptane was distilled off from the aqueous layer. Specifically, 70 mL of water co-distilled with heptane. After distilling off the heptane, tetrahydrofuran (THF; 1200 mL) was added to the aqueous layer and the resulting mixture was stirred vigorously at room temperature for 16 hours for slow hydrolysis. Monitoring via gas chromatography indicated the presence of some unreacted acid chloride. Addition water (150 mL) was then added to speed up the hydrolysis and the reaction was monitored by gas chromatography to ensure completion. The THF was then distilled off yielding a biphasic (water and oil) reaction mixture. Seeds were then added and the reaction was allowed to reach room temperature so that a heavy solid comes out. Water (250 mL) and acetonitrile (500 mL) were added to keep the suspension stirrable as the suspension was stirred for 2 hours at room temperature. The solid was then filtered off and washed with acetonitrile (2X250 mL). This filtrate contained the first crop of a- bromotricyclo [3.3. 1. 137] DECANE-L-ACETIC acid (Formula 0) ; 264 grams with AP 95 in 66% yield after DRYING IN VACUO at room temperature. The mother liquor (113 gram residue) was then triturated the residue with water and acetonitrile (250 mL/250 mL) for 1-2 hours at room temperature. The reaction was then filter and the solid dried to obtain a second crop of a-bromotricyclo [3.3. 1. 137] DECANE-L-ACETIC acid (Formula O) ; 64 grams with AP 90 in 16 % yield.
References:
WO2004/52850,2004,A2 Location in patent:Page 53
![Tricyclo[3.3.1.13,7]decane-1-acetyl chloride, α-bromo-](/CAS/20210305/GIF/98398-65-3.gif)
98398-65-3
0 suppliers
inquiry

59768-70-6
19 suppliers
$283.00/100mg