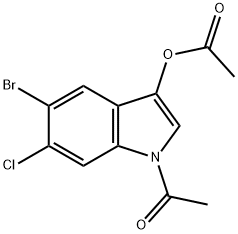
1-Acetyl-5-broMo-6-chloro-1H-indol-3-yl acetate synthesis
- Product Name:1-Acetyl-5-broMo-6-chloro-1H-indol-3-yl acetate
- CAS Number:108847-96-7
- Molecular formula:C12H9BrClNO3
- Molecular Weight:330.56
![Benzoic acid, 5-bromo-2-[(carboxymethyl)amino]-4-chloro-](/CAS/20200611/GIF/103029-90-9.gif)
103029-90-9
0 suppliers
inquiry
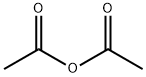
108-24-7
5 suppliers
$14.00/250ML

108847-96-7
41 suppliers
$60.00/1g
Yield: 68%
Reaction Conditions:
with sodium acetate at 135 - 140; for 0.416667 h;
Steps:
1.3 (3) Synthesis of 1-acetyl-5-bromo-6-chloro-3-indole ethyl ester (IV)
N- (4-bromo-5-chloro-2-carboxy) phenylglycine (III; 5.000 g, 16.21 mmol)Anhydrous sodium acetate(5.318 g, 64.83 mmol, 4.0 equiv.)And placed in 150mL two-necked round bottom flask,Acetic anhydride (75 mL) was added and placedIn an oil bath preheated to about 150 ° C,The reaction was stirred at 135-140 ° C until no more carbon dioxide was generated (about 25 min)Then remove,cool down,Add more ice water mixture,Stirring in an ice-water bath or standing until the oil disappears,Suction filtration,Saturated sodium bicarbonate to neutral,Wash thoroughly with water again,Pumping dry,A small amount of cold methanol washing,Ethanol - water recrystallization,After fully vacuum drying,The desired target 1-acetyl-5-bromo-6-chloro-3-indole ethyl ester (IV; 3.644g, 68% yield) was obtained.
References:
Guangdong Microbiology Institute (Guangdong Microbiology Analysis To Detect Center);Guangdong Huankai Microbiology Technology Co., Ltd.;Wu Qingping;Wei Xianhu;Zhang Jumei;Chen Moutong;Lu Mianfei;Cai Zhihe;Xue Liang;Wang Juan CN106986809, 2017, A Location in patent:Paragraph 0035; 0036; 0037; 0038
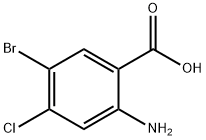
50419-88-0
74 suppliers
$35.00/250mg

108847-96-7
41 suppliers
$60.00/1g
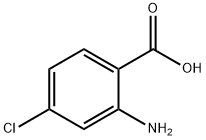
89-77-0
421 suppliers
$6.00/10g

108847-96-7
41 suppliers
$60.00/1g