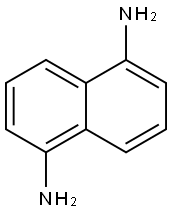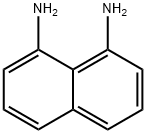
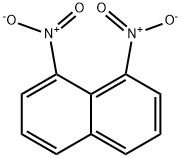
1,8-Diaminonaphthalene synthesis
- Product Name:1,8-Diaminonaphthalene
- CAS Number:479-27-6
- Molecular formula:C10H10N2
- Molecular Weight:158.2
602-38-0
0 suppliers
$68.09/1g

479-27-6
367 suppliers
$6.00/10g
Yield:479-27-6 96.46%
Reaction Conditions:
with iron(III) chloride hexahydrate;pyrographite;hydrazine hydrate;sodium hydroxide in ethanol;water at 65 - 90; pH=8 - 12;Autoclave;Industrial scale;Solvent;Temperature;
Steps:
1 Example 1: Preparation of 1,8-diaminonaphthalene
to the with a stirrer, thermometer, reflux condenser, of the storage tank is dropping 5000L enamel reaction in the cauldron throws Canada prefabricated catalyst 70 kg, tide b nitronaphthalenesulfonic (wherein two nitronaphthalenesulfonic the quality of 700 kg) and solvent ethanol 2500L, and then opens the stirrer, stirring state temperature to the material in the next cauldron 65 °C -75 ° C (this embodiment is 70 °C) the rear, at which temperature the slowly dropping 80.1% - 100% (in this embodiment is 100%) 520 kg of hydrazine hydrate, dropping time 5 hours, the reaction in cauldron material hydrated [...] to the to the 75 °C -90 ° C (this embodiment is 75 °C), at which temperature the thermal insulation reaction 3-8 hours (in this embodiment to 5 hours) to stop the reaction.The adding tide binitro the naphthalene passes through high performance liquid chromatography, hereinafter referred to as HPLC detection, the measured 1,8-dinitronaphthalene nitronaphthalenesulfonic of the total mass of the accounts for 95%, 1,5-dinitronaphthalene occupied nitronaphthalenesulfonic of the total mass of the 4.7%.The two nitro naphthalene tide second nitronaphthalenesulfonic in raw materials refer to two nitronaphthalenesulfonic refining comprising mixing the organic solvent used, is obtained after naphthalene nitration after being refined second nitronaphthalenesulfonic mixture of the product obtained by the refining operation drying; refined binitro naphthalene tidetide in the total mass of the solvent accounts for 5% - 15%. Binitro naphthalene tide contains the solvent may be methanol, ethanol, DMF or toluene.Nitration usually naphthalene obtained after the reaction is completed in two nitronaphthalenesulfonic product, 1,8-dinitronaphthalene is in a content of 55% the left and right, 1,5-dinitronaphthalene is in a content of 31% the left and right, other by nitrocompouind accounting for 4% the left and the right, water 10%. Therefore, the embodiment is the binitro naphthalene tidenaphthalene nitration containing the 1,5-dinitronaphthalene, 1,8-dinitronaphthalene and other nitro compound directly to an aqueous mixture of a refined to remove most 1, after 5-dinitronaphthalene, detection 1,8-dinitronaphthalene is the purity requirement of ≥ 95% as the preparation method of the embodiment of the use of tide b nitronaphthalenesulfonic raw material; here 1,8-dinitronaphthalene is the purity of the 1,8-dinitronaphthalene by nitrocompouind the content of the, water and the organic solvent does not take into account.The prefabricated method for prefabricated of the catalyst of the six-hydrated ferric chloride is dissolved in the water, the activated carbon is dispersed in suspension in aqueous alkaline solutions (in this embodiment of the alkaline aqueous solution is pH value is 8-12 of sodium hydroxide solution); then the iron chloride solution is poured into the activated carbon in suspension, ferric chloride and active carbon mass ratio is 1 the [...] 2-5 ; after stirring evenly mixed material filtering, drying the filter cake after the filtration of the catalyst, for use. Reaction b nitronaphthalenesulfonic with hydrazine hydrate in a molar ratio of 1 the [...] 3-4, the catalyst amount is binitro the naphthalene is wet second nitronaphthalenesulfonic quality of the material 2% - 10%.Except ethanol solvent, also can be methanol, DMF, or methanol, ethanol, DMF respectively mixed solvent with toluene.(2) after the step (1) reaction of the material cooling to 40 °C then press, filter press with a small amount of ethanol to obtain catalyst is subjected to a washing, washing the filtrate and as the feed liquid processing. (3) the step (2) of the liquid into the distillation still recovering desolution of the solvent under reduced pressure, decompression desolution of the solvent into the obtained after processing in the rectifying tower, recovering the solvent can be reused. The above-mentioned decompression desolution of the parameter is: pressure -0.050-0.070 MPa, stirring 50 °C -80 °C.The above-mentioned decompression desolution of the distillation of the solvent, the plate number of rectifying tower 60, feed liquid feed temperature is 30 °C, the feed plate as the section 30 block; overhead reflux ratio is 1 the [...] 6, the overhead condenser pressure is 0.098 MPa; recovery of ethanol from the top of the tower. (4) the desolvation (3) to the step of adding liquid 50 °C -80 ° C (this embodiment is 75 °C) hot water wash layered, the upper layer is an aqueous phase, the lower for material liquid phase, the hot water of the desolvation volume is the volume of the feed liquid 1-5 times (in this embodiment is 3 times).
References:
CN103664645,2016,B Location in patent:Paragraph 0024-0037
6364-17-6
7 suppliers
inquiry

479-27-6
367 suppliers
$6.00/10g
86-57-7
13 suppliers
$14.00/5g

479-27-6
367 suppliers
$6.00/10g
569-42-6
146 suppliers
$10.00/1g
![2-Phenyl-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine](/CAS/20210111/GIF/24341-81-9.gif)
24341-81-9
5 suppliers
inquiry
![Naphtho[1,8-de]-1,3,2-dioxaborin, 2-phenyl-](/CAS/20240320/GIF/60548-84-7.gif)
60548-84-7
0 suppliers
inquiry

479-27-6
367 suppliers
$6.00/10g