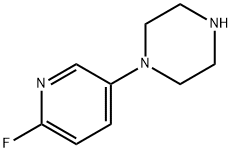
1-(6-fluoropyridin-3-yl)piperazine synthesis
- Product Name:1-(6-fluoropyridin-3-yl)piperazine
- CAS Number:1121610-07-8
- Molecular formula:C9H12FN3
- Molecular Weight:181.21
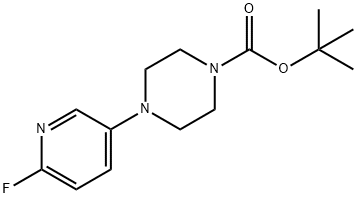
501126-13-2
1 suppliers
inquiry

1121610-07-8
5 suppliers
inquiry
Yield:1121610-07-8 500 mg
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 5; for 3 h;
Steps:
10b 10b. Preparation of Compound 66
65 (650 mg, 2.31 mmol) was dissolved in dichloromethane (10 mL) and cooled to 5 C. 5 mL solution of 20% TFA in DCM was added dropwise to the reaction mass. The reaction mixture was stirred for 3 h. Thereafter the reaction mixture was diluted with excess DCM (100 mL) and washed with water wash (2 x 50 mL) followed brine (50 mL). The organic layer was dried over sodium sulfate and concentrated under vacuum to give 500 mg of the desired product. LC-MS: m/z calcd for C9Hi2FN3, 181.21 , found 181.7 (M+H)+. *H NMR (500 MHz, DMSO-d6): δΗ 3.26 (4H, m, NCH2CH2N), 3.38 (4H, m, NCH2CH2N), 7.1 (1H, m, ArCH), 7.7 (1H, m, ArCH), 7.9 (1H, s, ArCH) and 9.0 (1H, s, NH)
References:
WO2013/90497,2013,A1 Location in patent:Page/Page column 49
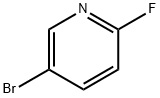
766-11-0
454 suppliers
$6.00/5g

1121610-07-8
5 suppliers
inquiry
