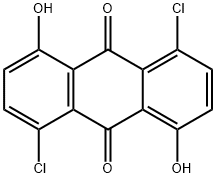
1,5-dichloro-4,8-dihydroxyanthraquinone synthesis
- Product Name:1,5-dichloro-4,8-dihydroxyanthraquinone
- CAS Number:6837-97-4
- Molecular formula:C14H6Cl2O4
- Molecular Weight:309.101
117-12-4
166 suppliers
$11.00/25g

6837-97-4
7 suppliers
inquiry
Yield:6837-97-4 49.5%
Reaction Conditions:
with sulfuryl dichloride in nitrobenzene at 20; for 22 h;Heating / reflux;
Steps:
1 Example 1
Example 1: Synthesis and characterization of anthraquinone fluorophore 1-(6-amino-hexylamino)-5-chloro-4,8-dihydroxy-anthraquinone (KAYA1) 1-(6-amino-hexylamino)-5-chloro-4,8-dihydroxy-anthraquinone (KAYA1) is prepared from commercially available 1,5-Dihydroxy-anthraquinone in a three-step reaction. The starting compound is converted into 1,5-dichloro-4,8-dihydroxy-anthraquinone by treatment with sulfurylchloride in 49.5 % yield after recrystallization according to a procedure described in Allen, C.F.H., Frame, G.F., Wilson, C.V., The Structures Of The So Called Touluidine Blue, Journal of Organic Chemistry, 1941, (6), 732-749. Procedure: 1,5-Dihydroxy-anthraquinone (10 g, 42 mmol) is dissolved in nitrobenzene. After addition of 10 ml SO2Cl2 (0.123 mol) the mixture is refluxed for two hours. An additional amount of SO2Cl2 (10 ml) was added dropwise and the reaction mixture is refluxed for further 20 hours. The excess SO2Cl2 is removed by heating to 210°C. Then the crude 1,5-dichloro-4,8-dihydroxy-anthraquinone is filtered and washed first with methanol and then with ether. The red compound is recrystallized from anisole.
References:
EP1905801,2008,A1 Location in patent:Page/Page column 7,8