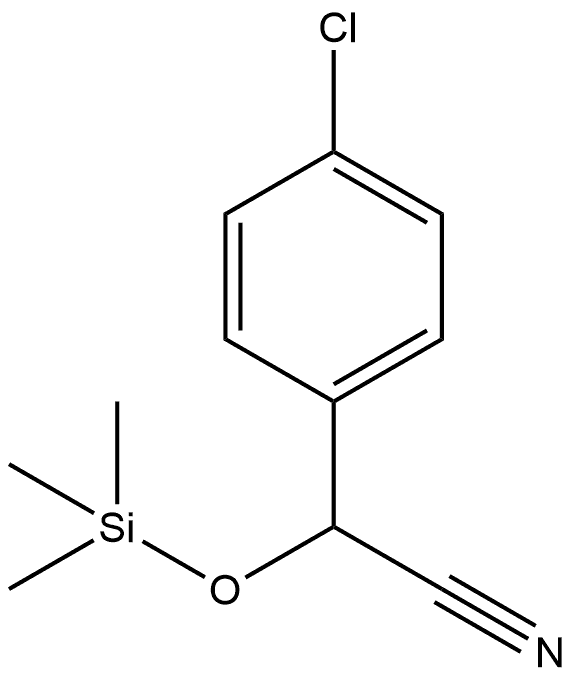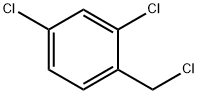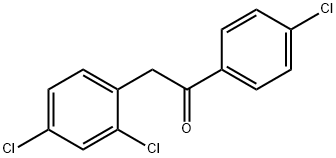
1-(4-Chlorophenyl)-2-(2,4-dichlorophenyl)ethanone synthesis
- Product Name:1-(4-Chlorophenyl)-2-(2,4-dichlorophenyl)ethanone
- CAS Number:654682-18-5
- Molecular formula:C14H9Cl3O
- Molecular Weight:299.58
Yield:-
Reaction Conditions:
Stage #1: (+/-)-2-(4-chlorophenyl)-2-(trimethylsiloxy)-acetonitrile;2,4-Dichlorobenzyl chloridewith lithium hexamethyldisilazane in tetrahydrofuran at -78 - 20;
Stage #2: with hydrogenchloride;water in tetrahydrofuran;
Steps:
1.A
A) 1-(4-Chlorophenyl)-2-(2,4-dichlorophenyl)ethanone; 32 g of LiHMDS are placed in 245 ml of THF at 0° C., and 40 g of (4-chlorophenyl) ((trimethylsilyl)oxy)acetonitrile are added slowly at -78° C., followed by 32.64 g of 2,4-dichloro-1-(chloromethyl)benzene. The temperature is allowed to return to AT overnight, and the reaction is then hydrolyzed with 170 ml of a 3N HCl solution, with gases being trapped in a solution of KOH (4N). After separation by settling out, the organic phase is evaporated, then taken up in DCM and agitated for 1 hour with 170 ml of NaOH (2N). The DCM is evaporated off and the expected compound is then crystallized from pentane. 38.6 g are obtained, Mp=119° C.
References:
US2006/189664,2006,A1 Location in patent:Page/Page column 6
1126-46-1
236 suppliers
$8.00/25g
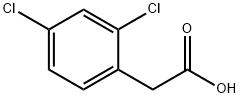
19719-28-9
388 suppliers
$7.00/10g

654682-18-5
23 suppliers
$305.00/1g

19719-28-9
388 suppliers
$7.00/10g
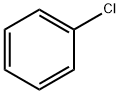
108-90-7
661 suppliers
$10.00/25G

654682-18-5
23 suppliers
$305.00/1g
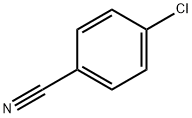
623-03-0
463 suppliers
$6.00/25g
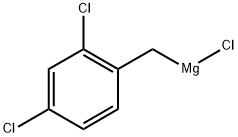
129752-86-9
9 suppliers
$301.00/100mL

654682-18-5
23 suppliers
$305.00/1g
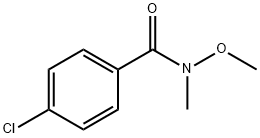
122334-37-6
88 suppliers
$8.00/1g

129752-86-9
9 suppliers
$301.00/100mL

654682-18-5
23 suppliers
$305.00/1g