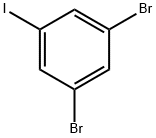
1,3-DIBROMO-5-IODOBENZENE synthesis
- Product Name:1,3-DIBROMO-5-IODOBENZENE
- CAS Number:19752-57-9
- Molecular formula:C6H3Br2I
- Molecular Weight:361.8
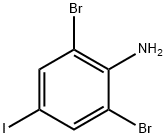
10527-69-2
16 suppliers
inquiry

19752-57-9
177 suppliers
$6.00/100mg
Yield:19752-57-9 136g
Reaction Conditions:
with hypophosphorous acid;sodium nitrite in ethanol;water at 20 - 25; for 4.16667 h;
Steps:
1 Example 1:
Preparation of 3,5-dibromo iodobenzene products: Add 2,6-dibromo-4-iodoaniline and hypophosphorous acid, at room temperature, 60 ml of sodium nitrite solution in a container.When no starting material by HPLC, the reaction was stopped.Direct filtration can be crude, the crude product was recrystallized from ethanol, to obtain the product as white needles.Detail of the product of step dibromo-3,5-iodobenzene was prepared as follows: wherein, charge amounts are as follows: 2,6-dibromo-4-iodoaniline: 188.4 g of, sodium nitrite: 34.5 g of, hypophosphite: 600 g of, ethanol: 700ml.In the reaction flask 1000ml 166.1g2,6- dibromo-4-iodo-aniline (0.5mol) and 600g of hypophosphorous acid, stirred for 10 minutes, and dispersed uniformly.Then dropping 34.5g sodium nitrite and 100ml water solution, violent exothermic reaction, the reaction temperature controlled water bath at 20-25 , about three hours after dropping, completion of the addition will be a lot of white solid precipitated.And then reacted at this temperature for 1 hour.The reaction was monitored by HPLC, when the raw material disappeared, the reaction was stopped, 20-25 filtered crude, the crude product was added to three 1000ml bottle, then add 700ml of absolute ethanol was heated to reflux (80 ) determined after the whole solution, cooling to 20-25 , filtered, and dried under reduced pressure to 60 deg.] C to give white needles 136g.As shown in Table 2 and 3,5-dibromo-2-iodobenzene shown HPLC plot, 3,5-dibromo iodobenzene map display content of 99.72 percent, 75.2 percent yield of the reaction.
References:
Shanghai Hua Xian New Material Technology Co., Ltd;Wu, Yuechu;Song, Wenzhi;Lin, Yafei CN105523884, 2016, A Location in patent:Paragraph 0014; 0016; 0017
626-39-1
464 suppliers
$5.00/5g

19752-57-9
177 suppliers
$6.00/100mg
626-40-4
203 suppliers
$39.00/5g

19752-57-9
177 suppliers
$6.00/100mg
17878-23-8
74 suppliers
$93.00/1 g

19752-57-9
177 suppliers
$6.00/100mg
540-37-4
443 suppliers
$6.00/5g

19752-57-9
177 suppliers
$6.00/100mg