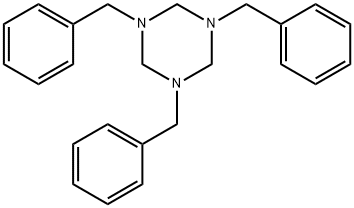
1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE synthesis
- Product Name:1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE
- CAS Number:2547-66-2
- Molecular formula:C24H27N3
- Molecular Weight:357.49

To a round-bottomed flask (125 mL) equipped with a reflux condenser was added theappropriate amine (0.05 mmol), toluene (40 mL) and formaldehyde (37%, 4.1 mL).The solution was brought to reflux using an external oil bath and kept stirring for 30min. Then, the toluene was evaporated under reduced pressure, and the residue wasdissolved in ethyl acetate and washed with a saturated aqueous solution of sodiumchloride. After evaporation of the solvent under reduced pressure in a rotaryevaporator, the residue was purified by silica gel column chromatography usinghexane/ethyl acetate 9:1 as the eluent.
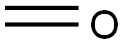
50-00-0
867 suppliers
$10.00/25g
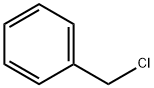
100-44-7
643 suppliers
$13.50/250G

2547-66-2
112 suppliers
$8.00/250mg
Yield:-
Reaction Conditions:
with ammonia in methanol at 40 - 70; under 600.06 Torr; for 6 h;
Steps:
1
Into a glass autoclave, 25.57 parts by weight of benzyl chloride (content: 99.0 wt%), 19.57 parts by weight of paraformaldehyde (content: 92 wt%) and 113.5 parts by weight of a 12 wt% ammonia/methanol solution were charged, and reacted under stirring at an internal temperature of 40°C for 3 hours, 50°C for 2 hours and 70°C for 1 hour. The maximum value of the internal pressure (gauge pressure) during the reaction was 0.08 MPa. The resulting reaction liquid and methanol rinse were transferred into a four-neck flask, subjected to reduced pressure to remove ammonium remaining in the reaction liquid, and further concentrated to remove methanol. To the residual liquid thus obtained was added 200 parts by weight of water, and methanol was distilled off together with water under reduced pressure. The residue was subjected to an extraction/separation treatment using 150 parts by weight of toluene to obtain 161.9 parts by weight of a toluene solution containing 1,3,5-tris(benzyl)-1,3,5-hexahydrotriazine. To the toluene solution were added 50 parts by weight of water, 68.5 parts by weight of a 24 wt% aqueous solution of hydroxylamine sulfate and 20.9 parts by weight of 35 wt% hydrochloric acid, and the mixture was stirred at room temperature for 1 hour. The mixture was adjusted to pH 13 by an addition of 103.6 parts by weight of a 27 wt% sodium hydroxide aqueous solution, and then subjected to an extraction treatment to obtain an organic layer and an aqueous layer. The separated aqueous layer was further extracted with 80 parts by weight of toluene, and the organic layer was combined with the previously obtained organic layer to obtain 233.3 parts by weight of a solution containing benzylamine. The yield of benzylamine was 85.6% (GC method; based on benzyl chloride).
References:
Sumitomo Chemical Company, Limited EP1961733, 2008, A1 Location in patent:Page/Page column 8
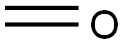
50-00-0
867 suppliers
$10.00/25g
100-46-9
486 suppliers
$5.00/5 g

2547-66-2
112 suppliers
$8.00/250mg

100-46-9
486 suppliers
$5.00/5 g
88891-55-8
9 suppliers
$270.00/100mg

2547-66-2
112 suppliers
$8.00/250mg

141-43-5
884 suppliers
$9.00/10g

75-09-2
1218 suppliers
$10.00/25 mL

100-46-9
486 suppliers
$5.00/5 g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2547-66-2
112 suppliers
$8.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2547-66-2
112 suppliers
$8.00/250mg