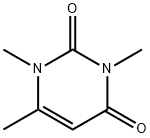
1,3,4-TRIMETHYL URACIL synthesis
- Product Name:1,3,4-TRIMETHYL URACIL
- CAS Number:13509-52-9
- Molecular formula:C7H10N2O2
- Molecular Weight:154.17
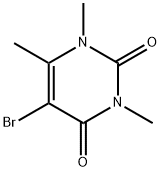
15018-59-4
29 suppliers
$80.00/250 mg

13509-52-9
53 suppliers
$45.00/250mg
Yield: 65%
Reaction Conditions:
with sulfuric acid;potassium iodide at 80; for 5 h;
Steps:
Reaction of 5-Iodo-1,3,6-trimethyluracil (4), 5-Bromo-1,3,6-trimethyluracil (5), or N-Bromo-5-bromo-6-hydroxymethyluracil (7) with KI in H2SO4 (5%) (General Method)
General procedure: A suspension of 4, 5, or 7 (1 mmol) and KI (0.83 g,5 mmol) in H2O (1.88 mL) at 80°C was treated dropwise with H2SO4 (98%, 0.05 mL, 1 mmol), stirred for 5 h at that temperature, cooled, treated with Na2S2O3·5H2O solution (10%), and extracted with CHCl3. The combined extracts were washed with distilled H2O, dried over Na2SO4, and evaporated to produce 6 (0.10 g, 68% yield from 4 and 0.10 g, 65% from 5).The physicochemical characteristics agreed with known data [8]. Compound 7 (0.27 g) was obtained as a mixture and a yellow amorphous powder, the main component of which was 5-bromo-6-iodomethyluracil (11).
References:
Chernikova;Spirikhin;Abdullin;Yunusov [Chemistry of Natural Compounds,2017,vol. 53,# 6,p. 1140 - 1143][Khim. Prir. Soedin.,2017,# 6,p. 968 - 971,4]
626-48-2
385 suppliers
$10.00/25 g
77-78-1
304 suppliers
$19.69/1ml

13509-52-9
53 suppliers
$45.00/250mg
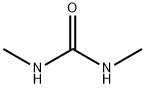
96-31-1
427 suppliers
$5.00/25g
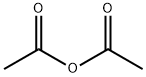
108-24-7
5 suppliers
$14.00/250ML

13509-52-9
53 suppliers
$45.00/250mg
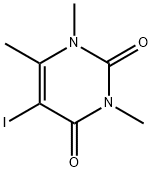
134039-54-6
11 suppliers
inquiry

13509-52-9
53 suppliers
$45.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

13509-52-9
53 suppliers
$45.00/250mg