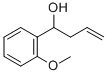
1-(2-METHOXYPHENYL)-3-BUTEN-1-OL 97 synthesis
- Product Name:1-(2-METHOXYPHENYL)-3-BUTEN-1-OL 97
- CAS Number:24165-67-1
- Molecular formula:C11H14O2
- Molecular Weight:178.23
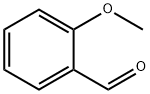
135-02-4
391 suppliers
$10.00/10g

1730-25-2
195 suppliers
$66.20/100ml

24165-67-1
12 suppliers
$474.00/1g
Yield:24165-67-1 100%
Reaction Conditions:
in tetrahydrofuran;diethyl ether at -70 - 20; for 2 h;
Steps:
17.1 Siep 1; l-(2-Methoxyphenyl)but-3-en-l-ol
Siep 1; l-(2-Methoxyphenyl)but-3-en-l-ol A soiuiion of 2-methoxybenzaldehyde (11 g, 81 mmol) in THF (300 mL) was cooled to below -70°C in a dry ice bath, Allylmagnesium bromide (i M in Et2G, 121 mL, 121 mmol) was added dropwise while keeping the temperature at or below -70°C. Once the addition was complete (about 2 h) the cooling bath was remo ved and the reaction mixture was allowed to warm to rt. The reaction mixture was then cooled to about 0 °C in an ice/water bath then saturated aqueous NH4CI (125 mL) was slowly added. Water (25 mL) was added and the layers were separated. The aqueous phase was extracted with EtOAc (125 mL) and the combined organics were washed with saturated aqueous NaCl (250 mL), dried over Na2S04, filtered, and concentrated under reduced pressure to give the title compound (14.4 g, 100%); NMR (400 MHz, Chloroform-i δ 7.39 - 7.30 (m, IH), 7.29 - 7.18 (m, 1H), 7.03 - 6.92 (m, IH), 6.91 - 6.84 (m, IH), 5.97 - 5.74 (m, IH), 5.23 - 5.04 (m, 2H), 5.04 - 4.91 (m, 1H), 3.85 (s, 3H), 2.69 - 2,34 (m, 2H).
References:
WO2016/168638,2016,A1 Location in patent:Page/Page column 50
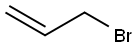
106-95-6
427 suppliers
$10.00/5g

135-02-4
391 suppliers
$10.00/10g

24165-67-1
12 suppliers
$474.00/1g
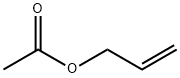
591-87-7
187 suppliers
$10.00/10g

135-02-4
391 suppliers
$10.00/10g

24165-67-1
12 suppliers
$474.00/1g
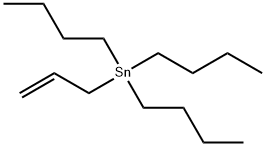
24850-33-7
188 suppliers
$12.00/5g

135-02-4
391 suppliers
$10.00/10g

24165-67-1
12 suppliers
$474.00/1g

591-87-7
187 suppliers
$10.00/10g
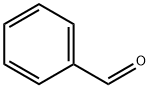
100-52-7
950 suppliers
$20.37/250g

24165-67-1
12 suppliers
$474.00/1g