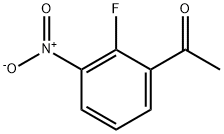
1-(2-fluoro-3-nitrophenyl)ethanone synthesis
- Product Name:1-(2-fluoro-3-nitrophenyl)ethanone
- CAS Number:873697-78-0
- Molecular formula:C8H6FNO3
- Molecular Weight:183.14
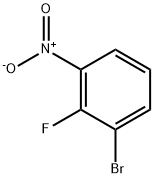
58534-94-4
188 suppliers
$10.00/1g
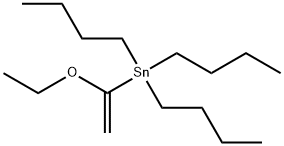
97674-02-7
236 suppliers
$15.00/1g

873697-78-0
42 suppliers
inquiry
Yield:873697-78-0 86%
Reaction Conditions:
with [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) in 1,4-dioxane at 90; for 4 h;
Steps:
8.1 Step 1. 1- (2-fluoro-3-nitrophenyl) ethanone
The dichlorobis (triphenylphosphine) palladium (II) (1.6g, 2.28mmol, 0.05equiv) was added to the two dioxanyl (100 mL) of 1-bromo-2-fluoro-3-nitrobenzene (10.0g, 45.6mmol, 1equiv) and tri-n-butyl (1-ethoxy-vinyl) stannane (15.4 mL, 45.6mmol, 1equiv) solution.The resulting cloudy solution was heated 4 hours at 90 , during which the formation of a dark brown solution.In TLC (30% MTBE / heptane) to confirm complete conversion, the reaction was cooled to room temperature.Was added KF (100mL) and ethyl acetate (100 mL), saturated solution, and the biphasic mixture stirred for 1 hour and filtered through celite, washed with ethyl acetate.The organic layer was separated over Na2SO4Sulfate, filtered, and evaporated to give a brown oil of the crude enol ether form.The crude product was dissolved in THF (50mL) and added 2NHCl (50mL).The reaction was stirred at room temperature for 1.5 hours.The reaction was then saturated with NaCl, and extracted with MTBE (2x150mL).Washed with brine (1x300mL) and the organic layer was washed over Na2SO4Sulfate, filtered, and evaporated to give the crude material was eluted through silica gel column chromatography crude material was purified with 0-40% ethyl acetate / heptane gradient solutions.Compound under reduced pressure, the product-containing fractions evaporated to give a yellow oil of 1- (2-fluoro-3-nitrophenyl) ethanone (7.1g, 86% yield)
References:
CN105228983,2016,A Location in patent:Paragraph 0697; 0698; 0699
943769-01-5
0 suppliers
inquiry

873697-78-0
42 suppliers
inquiry