1,2-DINITROGLYCERIN synthesis
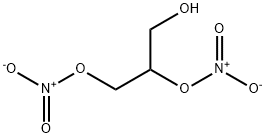
- Product Name:1,2-DINITROGLYCERIN
- CAS Number:621-65-8
- Molecular formula:C3H6N2O7
- Molecular Weight:182.09
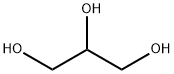
56-81-5
1699 suppliers
$5.00/25g
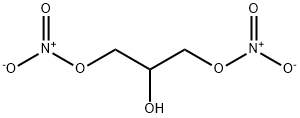
623-87-0
41 suppliers
$22.39/003-1ml

621-65-8
41 suppliers
$22.39/002-1ml
55-63-0
0 suppliers
$39.29/002-1ml
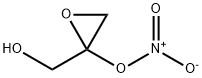
6659-62-7
6 suppliers
inquiry
Yield: 58 %Spectr. , 21 %Spectr.
Reaction Conditions:
Stage #1:glycerol with nitric acid at 22; for 0.141833 h;
Stage #2: with sodium hydroxide in water at 32; for 0.018 h;Temperature;Time;Concentration;
Steps:
5 Glycerol Nitration and DNG Intramolecular Condensation
The nitration and intramolecular condensation reactions were performed in series in the microfluidic reactor described in Table 4. Glycerol and nitric acid were injected into the T-joint under the process conditions discussed in Example 2 to produce DNG. The resonance tube containing DNG was then plumbed directly into the second T-joint where the DNG was mixed with NaOH and chemically transformed to GLYN. The glycerol and nitric acid were reacted at different process conditions and the reaction products were collected and analyzed by NMR. The specific process conditions and results for each of the reactions are shown in Tables 3-5. Table 3 includes the process variables (ratio of acid:glycerol, reaction temperature, flow rate, resonance time) that were tested for the nitration reaction and Table 5 includes the conversion results, which are expressed in mole percent (mol %) of the reaction products. The DNG and NaOH solution were reacted at different process conditions and the reaction products were collected and analyzed by NMR. Table 4 includes the process variables (NaOH concentration, temperature, flow rate, resonance time) that were tested and Table 5 includes the conversion results, which are expressed in mole percent (mol %) of the reaction products. GLYN was produced at a greater than about 58 mol % conversion from starting material. It is likely that additional optimization of the process conditions would further increase the reaction yield. The caustic induced transformation of DNG performed in the microfluidic reactor gave a high conversion to GLYN. Therefore, GLYN was successfully produced at a reasonable level of conversion.
References:
Northrop Grumman Innovation Systems, Inc.;Scavuzzo, Joseph J.;Mileham, Melissa L. US10562873, 2020, B1 Location in patent:Page/Page column 14; 15
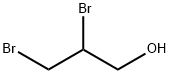
96-13-9
196 suppliers
$5.00/1g

621-65-8
41 suppliers
$22.39/002-1ml
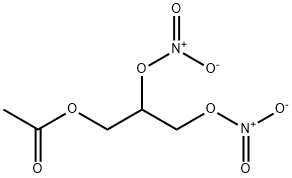
14739-14-1
1 suppliers
inquiry

621-65-8
41 suppliers
$22.39/002-1ml

56-81-5
1699 suppliers
$5.00/25g

623-87-0
41 suppliers
$22.39/003-1ml

621-65-8
41 suppliers
$22.39/002-1ml

55-63-0
0 suppliers
$39.29/002-1ml

56-81-5
1699 suppliers
$5.00/25g

623-87-0
41 suppliers
$22.39/003-1ml

621-65-8
41 suppliers
$22.39/002-1ml