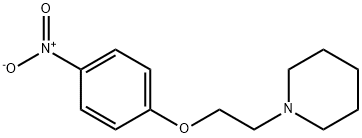
1-(2-(4-NITROPHENOXY)ETHYL)PIPERIDINE synthesis
- Product Name:1-(2-(4-NITROPHENOXY)ETHYL)PIPERIDINE
- CAS Number:92033-76-6
- Molecular formula:C13H18N2O3
- Molecular Weight:250.29
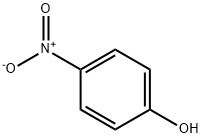
100-02-7
35 suppliers
$11.00/5G
2008-75-5
271 suppliers
$10.00/1g

92033-76-6
12 suppliers
$131.00/5g
Yield:92033-76-6 94%
Reaction Conditions:
Stage #1: 4-nitro-phenolwith potassium carbonate in N,N-dimethyl-formamide at 90;
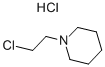
Stage #2: N-chloroethylpiperidine hydrochloride in N,N-dimethyl-formamide at 90; for 0.5 h;
Steps:
1-(2-(4-Nitrophenoxy)ethyl)piperidine (12).
4-Nitrophenol (2.00 g, 14.40 mmol) was dissolved in DMF (50 ml) under stirringin an Erlenmeyer flask. K2CO3 (5.96 g, 43.12 mmol) was added, and the mixture was heated to 90 °C on a magnetic stirrer. 16 (2.65 g,14.4 mmol) was added, and the solution was stirred for 30 min. The solution was cooled, water (100 ml) was added, and the solution was extracted with Et2O (4*40 ml). The organic phase was subjected to acid-base partitioning with 5% HCl (aq) (100 ml) and 5% NH4OH (aq) (150 ml), followed by extraction with Et2O (4* 60 ml).The solution was diluted with hexane (40 ml), dried with Na2SO4, filtered and evaporated. The crystalline residue was dried in vacuo to give 3.40 g (94%) of the title compound. The spectra were consistent with published data. mp, 62-64 °C; Lit., 63-64 °C.
References:
Norén, Rolf [Tetrahedron,2021,vol. 88,art. no. 132108]
110-89-4
0 suppliers
$15.96/100ML
13288-06-7
216 suppliers
$15.00/1g

92033-76-6
12 suppliers
$131.00/5g
3040-44-6
259 suppliers
$20.00/25g
350-46-9
528 suppliers
$10.60/1gm:

92033-76-6
12 suppliers
$131.00/5g

110-89-4
0 suppliers
$15.96/100ML
3383-72-0
143 suppliers
$25.00/1g

92033-76-6
12 suppliers
$131.00/5g

100-02-7
35 suppliers
$11.00/5G

92033-76-6
12 suppliers
$131.00/5g