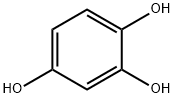
1,2,4-Benzenetriol synthesis
- Product Name:1,2,4-Benzenetriol
- CAS Number:533-73-3
- Molecular formula:C6H6O3
- Molecular Weight:126.11
Other production methods for 1,2,4-Benzenetriol are oxidation of resorcinol with hydrogen peroxide and Dakin oxidation of 2,4- or 3,4-dihydroxybenzaldehydes or 2,4- or 3,4-dihydroxyacetophenones with alkaline hydrogen peroxide solution.
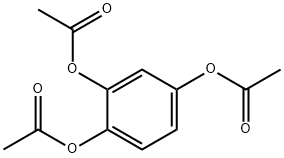
613-03-6
159 suppliers
$24.69/25g

533-73-3
296 suppliers
$10.00/1g
Yield:533-73-3 98%
Reaction Conditions:
with hydrogenchloride;water in methanol; for 7 h;Reflux;Reagent/catalyst;
Steps:
3 Synthesis of 1,2,4-Trihydroxybenzene from 1,2,4-Triacetoxybenzene
In an illustrative reaction, 670 g of 1,2,4-triacetoxybenzene (2.656 mol, 1 equivalent) was combined with 2.5 L of methanol, 1.5 L of deionized water, and 22 mL of 12 N hydrochloric acid (0.264 mol, 0.1 equivalents). The reaction mixture was then heated at reflux for 7 hours and cooled to room temperature over 14 hours. The reaction mixture was taken to dryness under reduced pressure to provide brown solids. 2 L of ethyl acetate was then added to the brown solids, which were then dissolved with heating. 200 g of solid NaHCO3 and 20 g of activated charcoal were then added. After heating to boiling for 30 minutes, the ethyl acetate solution was then allowed to partially cool. When the temperature reached approximately 45° C., the solids were removed by filtration and washed with an additional 400 mL of ethyl acetate. The filtrate was taken to dryness under reduced pressure to provide the product as a pale orange solid. After drying under vacuum, 314 g of product was collected (98%). FIG. 4 shows an illustrative 1H NMR spectrum in DMSO-d6 of 1,2,4-trihydroxybenzene synthesized by acidic hydrolysis of 1,2,4-triacetoxybenzene. Additional purification was realized through recrystallization from ethyl acetate in some instances.
References:
US2018/105544,2018,A1 Location in patent:Paragraph 0095
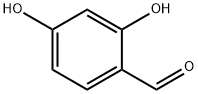
95-01-2
495 suppliers
$10.00/1g

533-73-3
296 suppliers
$10.00/1g
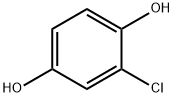
615-67-8
181 suppliers
$13.00/5g

533-73-3
296 suppliers
$10.00/1g
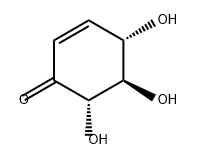
1190844-34-8
0 suppliers
inquiry

533-73-3
296 suppliers
$10.00/1g
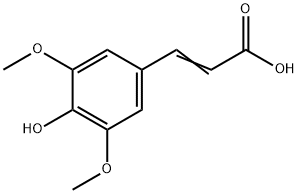
530-59-6
311 suppliers
$10.00/1g

533-73-3
296 suppliers
$10.00/1g