
1,1,1,2-TETRACHLOROETHANE synthesis
- Product Name:1,1,1,2-TETRACHLOROETHANE
- CAS Number:630-20-6
- Molecular formula:C2H2Cl4
- Molecular Weight:167.85
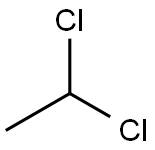
75-34-3
105 suppliers
$22.69/48602

79-00-5
215 suppliers
$15.00/10 mL

630-20-6
96 suppliers
$44.60/40335
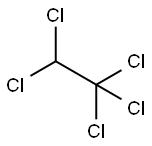
76-01-7
68 suppliers
$38.39/5ml-a
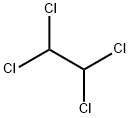
79-34-5
273 suppliers
$10.00/5g
Yield:-
Reaction Conditions:
with sulfuryl dichloride in chloroform at 175; under 760.051 Torr;Temperature;
Steps:
3 Simultaneous Gas-phase Dehydrochlorination and Chlorination of 1, 1-Dichloroethane
solution of 1 wt% 1 , 1 -dichloroethane in chloroform was prepared, and the solution divided into two portions. 4 wt% Sulfuryl chloride was added to a first portion and both portions were pumped independently at 10-30 liquid cc/hour into a vaporizer and reactor as shown in Figure 2. The reactor was 1 .09 cm ID and 25.4 cm long, packed with Y-type zeolite 1/16" extrudates. Reactor temperature was 125°C- 200°C and pressure was atmospheric pressure. The reactor effluent was cooled and condensed at 0°C and samples of the condensed effluent were analyzed by GC/MS.As shown in Figure 3, at a residence time of about 10 s and temperature of 175°C, most of the 1 , 1 , -dichloroethane without sulfuryl chloride reacted as shown in Table 1 below. With sulfuryl chloride present, 1 , 1 ,-dichloroethane completely reacted at these conditions as shown in Table 2 below. In Figure 3 and 4, the cases without sulfuryl chloride were for comparison only, and demonstrate that the dehydrochlorination reaction alone becomes equilibrium limited. This data also shows that chlorination needs to occur substantially simultaneously in order to overcome the cracking equilibrium and to provide essentially complete conversions.
References:
BLUE CUBE IP LLC;MYERS, John D.;GRANDBOIS, Matthew L. WO2017/53528, 2017, A1 Location in patent:Paragraph 0062; 0063
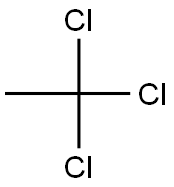
71-55-6
0 suppliers
$24.10/48614

630-20-6
96 suppliers
$44.60/40335
75-35-4
192 suppliers
$20.00/5 g

76-01-7
68 suppliers
$38.39/5ml-a

79-01-6
472 suppliers
$17.60/100ml

630-20-6
96 suppliers
$44.60/40335

79-34-5
273 suppliers
$10.00/5g

630-20-6
96 suppliers
$44.60/40335

75-35-4
192 suppliers
$20.00/5 g

630-20-6
96 suppliers
$44.60/40335