METHANE
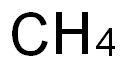
|
3 prices
Selected condition:
Brand
- American Custom Chemicals Corporation
- Sigma-Aldrich
Package
- 5MG
- 14L
- 24l
- ManufacturerAmerican Custom Chemicals Corporation
- Product numberGAS0000107
- Product descriptionMETHANE 95.00%
- Packaging5MG
- Price$500.52
- Updated2021-12-16
- Buy
- ManufacturerSigma-Aldrich
- Product number22562
- Product descriptionMethane (99.0%) 99.0%, cylinder of 14?L, analytical standard
- Packaging14L
- Price$242
- Updated2024-03-01
- Buy
- ManufacturerSigma-Aldrich
- Product number463035
- Product descriptionMethane electronic grade, ≥99.998%
- Packaging24l
- Price$2020
- Updated2024-03-01
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | GAS0000107 | METHANE 95.00% | 5MG | $500.52 | 2021-12-16 | Buy |
| Sigma-Aldrich | 22562 | Methane (99.0%) 99.0%, cylinder of 14?L, analytical standard | 14L | $242 | 2024-03-01 | Buy |
| Sigma-Aldrich | 463035 | Methane electronic grade, ≥99.998% | 24l | $2020 | 2024-03-01 | Buy |
Properties
Melting point :−183 °C(lit.)
Boiling point :−161 °C(lit.)
Density :0.716 g/mL at 25 °C(lit.)
vapor density :0.55 (vs air)
refractive index :1.0004
Flash point :-188 ºC
form :gas
pka :48(at 25℃)
Odor :odorless
Flame Color :(Light) blue
explosive limit :15%
Water Solubility :24.4mg/L(25 ºC)
Merck :13,5979
BRN :1718732
Dielectric constant :1.7(-173℃)
Stability :Stable. Extremely flammable - note low flash point; mixtures with air constitute an explosion hazard. Reacts violently with interhalogens. Incompatible with strong oxidizing agents, halogens, interhalogens, oxygen.
CAS DataBase Reference :74-82-8(CAS DataBase Reference)
EPA Substance Registry System :Methane (74-82-8)
Boiling point :−161 °C(lit.)
Density :0.716 g/mL at 25 °C(lit.)
vapor density :0.55 (vs air)
refractive index :1.0004
Flash point :-188 ºC
form :gas
pka :48(at 25℃)
Odor :odorless
Flame Color :(Light) blue
explosive limit :15%
Water Solubility :24.4mg/L(25 ºC)
Merck :13,5979
BRN :1718732
Dielectric constant :1.7(-173℃)
Stability :Stable. Extremely flammable - note low flash point; mixtures with air constitute an explosion hazard. Reacts violently with interhalogens. Incompatible with strong oxidizing agents, halogens, interhalogens, oxygen.
CAS DataBase Reference :74-82-8(CAS DataBase Reference)
EPA Substance Registry System :Methane (74-82-8)
Safety Information
| Symbol(GHS): |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||
| Precautionary statements: |
|
Description
Methane is a colorless, odorless, flammable hydrocarbon gas that is the simplest alkane. The root word, met, in methane is derived from the Greek root word methe meaning wine. Methylene was used in the early 19th century as the name for methanol, which is wood alcohol, CH3OH. Methylene comes from methe + hydē, the latter being the Greek word for wood, so methylene would mean wine from wood. Methanol got the names methylene and wood alcohol because it was discovered by Robert Boyle (1627–1691) in the 17th century by the destruction distillation of wood. Destructive distillation involves heating in the absence of air.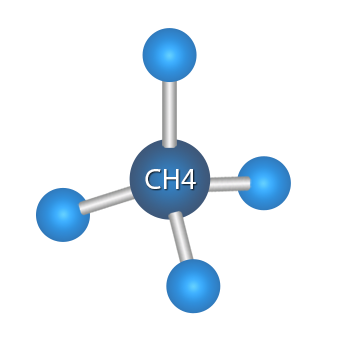
Methane is the first alkane and carries the suffix“ane” denoting an alkane, thus methe z + ane = methane. The carbon is at the center of the tetrahedron, which can be assumed to be an equilateral pyramid, with a hydrogen atom at each of the four corners of the tetrahedron.
Methane is the principal component of natural gas, with most sources containing at least 75% methane. Methane production occurs naturally through a process called methanogenesis. Methanogenesis involves anaerobic respiration by single-cell microbes collectively called methanogens.
Related product price
- ETHYLENE
$79-6745.2 - ETHANE
$269-2352.46 - Ferric acetylacetonate
$6-2359.48
Suppliers and manufacturers
Henan Tianfu Chemical Co.,Ltd.
Hubei xin bonus chemical co. LTD
Hefei TNJ Chemical Industry Co.,Ltd.
Shaanxi Dideu Medichem Co. Ltd
PT CHEM GROUP LIMITED
XIAMEN AMITY INDUSTRY AND TRADE CO., LTD.
Mainchem Co., Ltd.
Guangzhou Yuejia Gas Co., Ltd
Central China Special Gas (CCSG) Co., Ltd
Isotope (Xiamen) Industry and Trade Co., Ltd





