| Aladdin Scientific | |
|---|---|
| Country: | United States |
| Tel: | 400-620-6333 |
| E-mail: | sales@aladdinsci.com |
| QQ: | |
| Skype: | Chat Now! |
【Aladdin】White Catalyst
Release time: 2024-11-29
White Catalyst

Product Manager:Nick Wilde
Introduction
The "White catalyst" (B488782), developed by Professor M. Christina White and her team at the University of Illinois-Urbana, has proven to be an outstanding catalyst for the functionalization of allylic carbon centers.
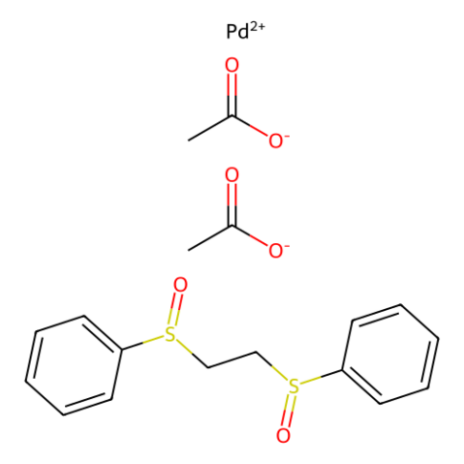
Figure 1.White Catalyst (B488782)
The bis-sulfoxide-Pd(II) catalyst is involved in a wide range of reactions, including both inter- and intramolecular allylic C-H oxidation, sequential allylic C-H oxidation followed by vinylic C-H arylation, and newly discovered allylic C-H amination. These versatile transformations facilitate the swift synthesis of various important intermediates, such as allylic carboxylates, macrocycles, and amino alcohol derivatives.
Advantages
● Stable catalyst in air
● Enables synthesis of complex, heteroatom-rich intermediates from basic starting materials
Representative Applications
Allylic C-H Oxidation
In 2004, the White group introduced a catalytic system for α-olefin allylic oxidation that was promoted by sulfoxide and involved Pd(II)/benzoquinonone/HOAc. The ratio of linear to branched allylic acetates produced in this system was influenced by the specific sulfoxide used in the reaction, whether it was DMSO or bis-sulfoxide ligands. Effective catalysts for the selective allylic C-H oxidation to yield branched allylic acetates included the "White catalyst" (1,2-Bis(phenylsulfinyl)ethane Palladium(II) Diacetate) or phenyl vinyl sulfoxide in the presence of palladium acetate.
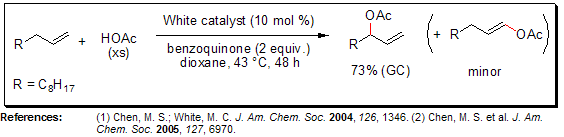
Macrolactonization via Allylic C-H Oxidation
The intermolecular reaction involving linear ω-alkenoic acids provides an excellent approach for synthesizing macrolactones, offering an alternative to the commonly used hydroxyacid lactonizations. This novel macrolactonization method has a wide range of applicability, as it can accommodate aryl, alkyl, and α,β-unsaturated carboxylic acids as nucleophiles, with the latter undergoing the reaction without olefin isomerization. Furthermore, this macrolactonization process is highly tolerant of polar functionalities, such as esters, imides, and amides.

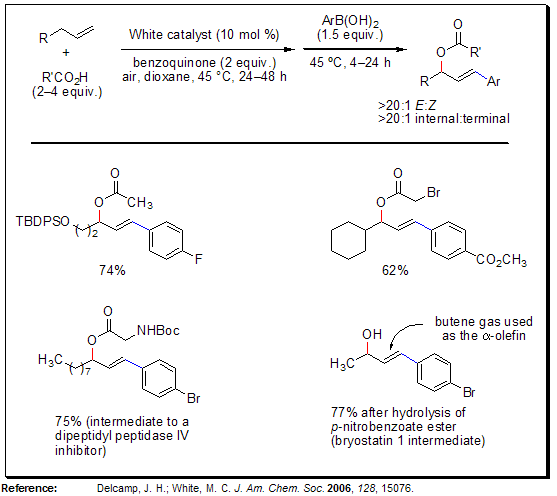
Sequential Allylic C-H Oxidation/Vinylic C-H Arylation
The process of allylic C-H oxidation followed by vinylic C-H arylation of α-olefins efficiently produces E-arylated allylic esters with exceptional regio- and stereoselectivity. This approach facilitates swift access to highly functionalized fragments essential for synthesizing complex molecules, starting from inexpensive and readily available materials such as α-olefins, carboxylic acids, and arylboronic acids. In contrast to the typical Heck reaction, which operates under basic, reductive conditions, this Pd(II)-catalyzed C-H arylation takes place under mildly acidic, oxidative conditions. This allows for the utilization of ortho-substituted and electron-deficient arylboronic acids. Moreover, the reaction conditions are compatible with a diverse array of polar functionalities present in the α-olefin component.
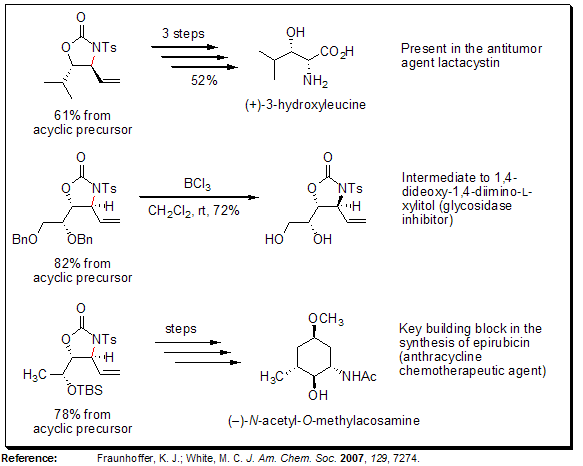
Allylic C-H Amination-Preparation of syn-1,2-Amino Alcohol Derivatives
White and his team have showcased the pioneering palladium-catalyzed allylic C-H amination reaction using a bis-sulfoxide-Pd(II) catalyst, which successfully converts N-tosylcarbamate precursors into functionalized oxazolidinones. This amination process exhibits selectivity for the anti-oxazolidinone diastereomer, enabling swift access to medically significant syn-1,2 amino alcohol structures.
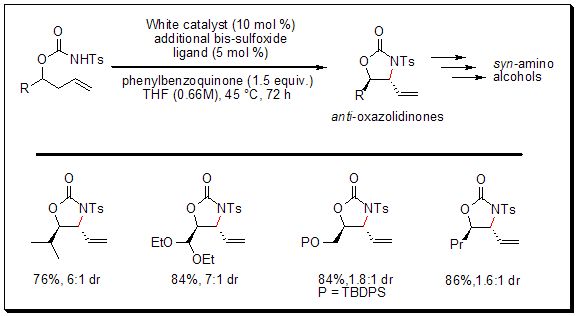
As depicted below, anti-oxazolidinones serve as versatile intermediates in the synthesis of various biologically active compounds, such as antitumor agents, enzyme inhibitors, and chemotherapeutic drugs. Notably, this approach simplifies the synthesis of heteroatom-rich molecules by minimizing the total number of synthetic steps (including functional group modifications) and facilitating the late-stage incorporation of nitrogen functionality.
Aladdin:https://www.aladdinsci.com

