What is Methylglyoxal?
Feb 26,2020
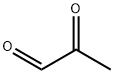
Methylglyoxal (MG, C3H4O2, CAS registry No. 78-98-8) is also known as 2-oxopropanal, pyruvaldehyde, pyruvic aldehyde, 2-ketopropionaldehyde, acetylformaldehyde, propanedione, or propionaldehyde, which is a clear yellow slightly viscous liquid with a pungent odor which polymerizes readily and forms a variety of cyclic and acyclic structures. It is faintly acidic to litmus. Its melting point is 25 oC, and flash point is 9 oC. The solubility of methylglyoxal is more than 10 g/100 mL water at 17 ºC. In water, MG is present mostly in the mono and dihydrate forms, while non hydrated MG is only present in traces.
Methylglyoxal is a highly reactive alpha-oxoaldehyde formed endogenously in numerous enzymatic and nonenzymatic reactions. Methylglyoxal can change from less reactive non carbonyl form to more reactive carbonyl and dicarbonyl forms and vice versa in the presence of some organic compounds. These changes are dependent on temperature and the amount of water, and can strongly influence MG reactivity in different parts of cells or tissues. It modifies arginine and lysine residues in proteins forming advanced glycation end-products such as Nδ-(5-methyl-4-imidazolon-2-yl)-L-ornithine (MG-H1), 2-amino-5-(2-amino-5-hydro-5-methyl-4-imidazolon-1-yl)pentanoic acid (MG-H2), 2-amino-5-(2-amino-4-hydro-4-methyl-5-imidazolon-1-yl)pentanoic acid (MG-H3), argpyrimidine, Nδ-(4-carboxy-4,6-dimethyl-5,6-dihydroxy-1,4,5,6-tetrahydropyrimidine-2-yl)-L-ornithine (THP), Nε-(1-carboxyethyl)lysine (CEL), MG-derived lysine dimer (MOLD), and 2-ammonio-6-({2–[4-ammonio-5-oxido-5-oxopently)amino]-4-methyl-4,5-dihydro-1H-imidazol-5-ylidene}amino)hexanoate (MODIC)[1], which have been identified in vivo and are associated with complications of diabetes and some neurodegenerative diseases.
Many food products, beverages, water, rain, clouds, fog water, and urban atmosphere as well as cigarette smoke represent exogenous sources of methylglyoxal. The origins of MG in food and beverages are sugars, the products of the Maillard reaction, lipids and microorganisms formed during industrial processing, cooking, and prolonged storage[1]. In vivo MG can be formed in many enzymatic and nonenzymatic pathways. Enzymatic pathways include reactions catalyzed by triosephosphate isomerase, cytochrome P450 2E1, myeloperoxidase, and aminooxidase, whereas nonenzymatic pathways include decomposition of dihydroxyacetone phosphate (DAP), the Maillard reaction, oxidation of acetol, and lipid peroxidation. Fasting and metabolic disorders such as diabetes can induce an increase in the formation of this highly reactive α-oxoaldehyde[1].
Several enzymes are involved in the detoxification of methylglyoxal and make a network of four recognized catabolism pathways, as follows: the glyoxalase system, aldose reductase (ALR), betaine aldehyde dehydrogenase, and 2-oxoaldehyde dehydrogenase (2-ODH)[1]. The glyoxalase system is a metabolic pathway that catalyzes the detoxification of MG to D-lactate. It consists of two enzymes, glyoxalase I and glyoxalase II, and a catalytic amount of reduced glutathione (GSH). Glyoxalase I (EC 4.4.1.5) catalyzes the formation of S-D-lactoylglutathione hemithioacetal formed nonenzymatically from GSH and MG. Glyoxalase II (EC 3.2.1.6) catalyzes the hydrolysis of S-hydroxyacetylglutathione to D-lactate with regeneration of GSH consumed in the reaction catalyzed by glyoxalase I. ALR (EC 1.1.1.21, ALR2, AKR1B1) catalyzes the NADPH-dependent reduction of MG into lactaldehyde in the presence of GSH and into acetol in the absence of GSH. Betaine aldehyde dehydrogenase catalyzes the NAD-dependent oxidation of MG into pyruvate. 2-ODH catalyzes methylglyoxal oxidation into pyruvate. It may represent an important liver detoxification enzyme for protection against methylglyoxal.
Methylglyoxal in biological samples can be quantified by HPLC or GC methods with preliminary derivatization into more stable chromophores and/or fluorophores, or derivatives suitable for determination by MS by use of diamino derivatives of benzene and naphthalene, 6-hydroxy-2,4,5-triaminopyrimidine, cysteamine, and o-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine[1]. The methods include three basic steps: deproteinization, incubation with derivatization agent, and chromatographic analysis with or without preliminary extraction of the formed products.
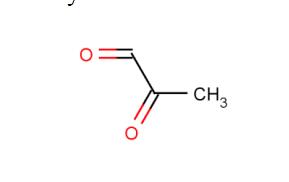
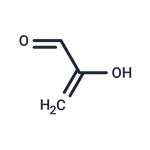
Fig 1. Chemical structure formula of methylglyoxal
References
[1]. Nemet, I.; Varga-Defterdarovic, L.; Turk, Z., Methylglyoxal in food and living organisms. Molecular nutrition & food research 2006, 50 (12), 1105-17.
- Related articles
- Related Qustion
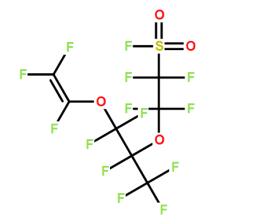
Perfluoro(8-cyano-5-methyl-3,6-dioxaoct-1-ene), 2,2,3,3,4,4,5,5,6,6-decafluoro-6-[(1,2,2-trifluoroethenyl)oxy] hexanenitrile MV5CN were successfully used in terpolymers to allow the crosslinking(by formation of stable triazine rings).....
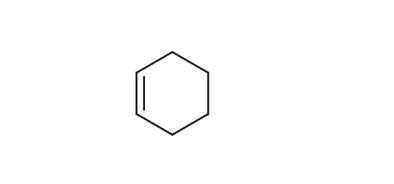
Feb 26,2020Organic Raw MaterialCyclohexene with an active double bond is a cheap, abundant, and easily accessible raw material in industry, and can be synthesized by hydrogenation of benzene.....
Feb 26,2020Organic ChemistryMethylglyoxal
78-98-8You may like
- Methylglyoxal
-

- $75.00 / 1kg
- 2024-11-19
- CAS:78-98-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Pyruvic aldehyde
-
- $50.00 / 1mL
- 2024-11-08
- CAS:78-98-8
- Min. Order:
- Purity:
- Supply Ability: 10g
- Methylglyoxal
-
- $100.00 / 25KG
- 2024-10-31
- CAS:78-98-8
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 200TON