Voglibose: Overview, Properties and Synthesis from Valiolamine
Nov 13,2024
General Description
Voglibose, a pharmaceutical compound derived from valiolamine, exhibits potent inhibitory activity against α-glucosidases, making it effective in addressing hyperglycemia and related disorders. It demonstrates strong anti-obesity and anti-diabetic activities, with clinical trials highlighting its efficacy in reducing postprandial blood glucose levels. Voglibose also shows promising results in lowering triglyceride levels and elevating HDL cholesterol. Compared to acarbose, it has fewer adverse effects, positioning it as a valuable therapeutic option for individuals with diabetes and metabolic disorders. Voglibose's synthesis from valiolamine involves several key steps, showcasing its complex yet meticulously orchestrated process.

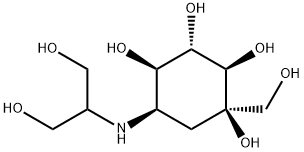
Figure 1. Voglibose
Overview
Voglibose is a pharmaceutical compound that has garnered significant attention due to its wide-ranging therapeutic and pharmacological properties. Structurally, it is an N-substituted derivative of valiolamine, a branched-chain aminocyclitol, with its N-substituted moiety derived from glycerol. Voglibose exhibits potent inhibitory activity against α-glucosidases, making it effective in addressing hyperglycemia and related disorders. Notably, voglibose demonstrates strong anti-obesity and anti-diabetic activities, positioning it as a potent glucosidase inhibitor and a valuable treatment option for non-insulin-dependent diabetes mellitus (NIDDM) in several Asian countries, including Japan, China, and Korea. Comparative studies have shown voglibose to be 20-30 times more potent in inhibiting semipurified porcine small intestine disaccharides compared to acarbose, a typical α-glucosidase inhibitor. Clinical trials have further highlighted the efficacy of voglibose in reducing postprandial blood glucose concentration in both animal models and human subjects with diabetes mellitus. Additionally, voglibose treatment has resulted in a significant decline in triglyceride levels and an elevation of high-density lipoprotein (HDL) cholesterol and apolipoprotein A-1. Importantly, compared to acarbose, voglibose has demonstrated fewer adverse symptoms, showing a promising safety profile in clinical trials. In summary, voglibose's multifaceted benefits, including its potent α-glucosidase inhibition, efficacy in managing postprandial blood glucose levels, and favorable safety profile make it a valuable therapeutic option for individuals with diabetes and related metabolic disorders. 1
Properties
Voglibose is a compound with the molecular formula C10H21NO7 and a molecular weight of 267.28. It exhibits certain physico-chemical properties, such as being easily soluble in water and acetic acid, while it is not easily soluble in methanol and ethanol and almost insoluble in ether. With a melting point of about 166°C, voglibose has a sweet taste but no smell. The 1H NMR spectra of voglibose in both hydrochloride and non-hydrochloride forms reveal its structural characteristics. One of the key properties of voglibose lies in its ability to inhibit intestinal α-glucosidases, which play a role in the digestion of disaccharides like maltose and sucrose. This inhibitory effect makes voglibose significant for the treatment of NIDDM (non-insulin-dependent diabetes mellitus). Voglibose's inhibitory effects on porcine maltase and sucrase have been found to be higher compared to other related compounds, demonstrating its potential for therapeutic applications. The introduction of a hydroxyl group into specific positions on the alkyl unit has been observed to enhance the inhibitory activities of voglibose, particularly against porcine maltase. Furthermore, voglibose has shown strong inhibitory activity against disaccharidases, displaying no α-amylase and β-D-glucosidase inhibitory activity in vitro. This strong inhibitory activity can be attributed to the similar three-dimensional positioning of voglibose's structure to that of sucrose and maltose, as evidenced by NMR spectral data and the formation of an intramolecular hydrogen bond. Given its ease of synthesis and safety of potential metabolites in the body, voglibose was selected for further biological evaluation over other N-substituted valiolamine derivatives, making it a promising candidate for the treatment of NIDDM. 2
Synthesis from Valiolamine
Voglibose was first synthesized from valiolamine. Valiolamine, (1S)-[1(OH), 2,4,5/1]-5-amino-1-hydroxymethyl-1,2,3,4-cyclohexanetetrol, was isolated from the fermentation broth. Since the discovery of valiolamine, five total syntheses of valiolamine have been reported with one starting from a Diels-Alder (furanacrylic acid) cycloadduct, one from D-glucose via a Ferrier rearrangement, the third one from 2, 3, 4, 6- tetra-Obenzyl-D-glucono-1, 5-lactone employing an aldol reaction as the key step, the fourth one from (-)-quinic acid via the approach in the facile synthesis, a novel acetyl migration and internal displacement reaction involving neighboring group participation, and the last one from validamine and valienamine via the stereoselective conversion.
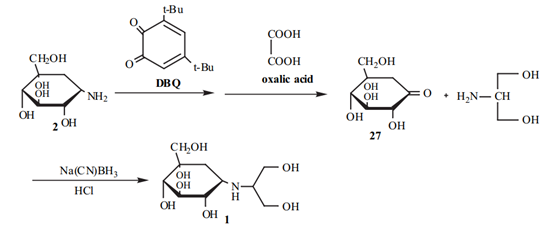
Scheme 1. Synthesis of Voglibose from Valiolamine.
In one method, valiolamine is oxidatively deamined to produce valiolone 27 with oxidizing agents in alcohol at 50°C. Oxidizing agents, which are known to be effective in converting amines to imines, can be used. 3, 5-Di-t-butyl-1, 2-benzoquinone (DBQ), nicotinic aldehyde in the presence of a base and benzothiazole-2-aldehyde were used as oxidizing agents. Then, hydrolysis preceded using oxalic acid and conditions for hydrolyzing an imine to a ketone at pH 5.0. After the achievement of valiolone, voglibose could be prepared by reacting valiolone with 2-amino-1, 3-propanediol (serinol) in the presence of a reducing agent, Na(CN)BH3, with or without an acid catalyst (e.g., acetic acid, HCl), according to the reaction (Scheme 1).2
References:
[1] KAKU K. Efficacy of voglibose in type 2 diabetes.[J]. Expert Opinion on Pharmacotherapy, 2014, 15 8. DOI:10.1517/14656566.2014.918956.
[2] XIAOLONG CHEN Y S Yuguo Zheng. Voglibose (Basen, AO-128), one of the most important alpha-glucosidase inhibitors.[J]. Current medicinal chemistry, 2006, 13 1. DOI:10.2174/092986706789803035.
- Related articles
- Related Qustion
- Voglibose: mechanism of action, pharmacokinetics and safety Aug 25, 2023
Voglibose is a α-glucosidase inhibitor for type 2 diabetes that can regulate blood glucose without causing hypoglycemia and may cause gastrointestinal side effects.
- Role of voglibose in prevention of type 2 diabetes Aug 19, 2019
Voglibose (INN and USAN, trade name Voglib, marketed by Mascot Health Series) is an alpha-glucosidase inhibitor used for lowering post-prandial blood glucose levels in people with diabetes mellitus. Voglibose delays the absorption of glucose thereby reducing the risk of macrovascular complications. Voglibose is a research product of Takeda Pharmaceutical Company, Japan's largest pharmaceutical company. Voglibose was first launched in 1994, under the trade name BASEN, to improve postprandial hyperglycemia in diabetes mellitus.