Synthesis and Application of 1, 5-Diaminopentane
Nov 21,2022
General description
1, 5-Diaminopentane is a colorless, viscous, smoky liquid with special smell. It is easily soluble in water, ethanol, but insoluble in ether. It can be solidified and crystallized by deep freezing, but it can be melted into liquid at room temperature. It has a bad smell of hexahydropyridine, smokes in the air, and forms dihydrate. It can be used as intermediate of organic synthesis, curing agent of epoxy resin, preparation of polymers, and also used in biological research.
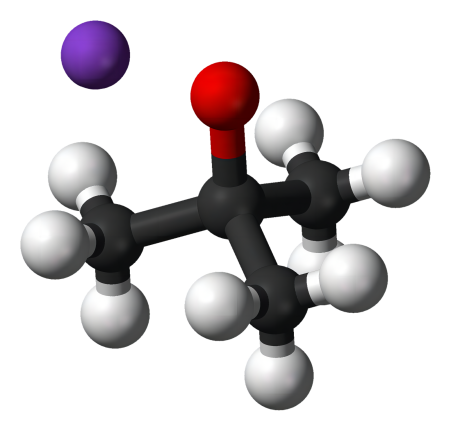
Fig. 1 The structure of 1,5-Diaminopentane.
Synthetic routes
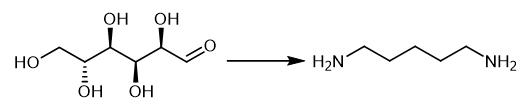
Fig. 2 The synthetic method 1 of 1,5-Diaminopentane.
Perform the cadaverine production with E. coli DFC1001 derived from the L-lysine producer E. coli NT1003. Cultivate E. coli DFC1001 contains cadA gene encoding lysine decarboxylase, and in Luria-Bertani (LB) medium (contained 50 μg/mL streptomycin) for 12 hours and incubate in 37°C with shaking at 200 rpm. Add 1 mL of seed culture into 300 mL of fermentation medium which contained (per liter) 30 g glucose, 10 g (NH4)2SO4, 5 g yeast extract, 0.5 g KCl, 1 g MgSO4·7H2O, 32 mg FeSO4·7H2O, 32 mg MnSO4·H2O, 86 mg ZnSO4·7H2O, 77 mg CuSO4, 60 mg/L vitamin B1, 10 mg nicotinamide, 30 μg biotin, 0.3 g L-threonine, 0.1 g L-methionine, after incubation. Observe the fermentation medium for strains additionally contained antibiotics (50 μg/mL streptomycin) and incubated in 37°C with shaking at 200 rpm. Keep the pH constant at 7.0 by automated addition of NH4OH (30%, v/v). Maitain the dissolved oxygen above 15% by variation of the stirrer and aeration rate to obtain cadaverine [1].
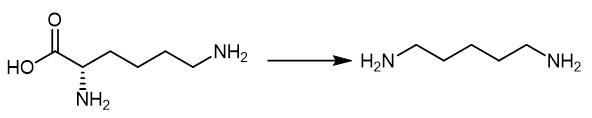
Fig. 3 The synthetic method 2 of 1,5-Diaminopentane.
Perform the reaction on the optimal conditions determined by following experiment. Use 100 mM potassium phosphate buffer (pH 7) as the phosphate donor to the mixture. Add 5 mM MgCl2 also as metal ion cofactor to the mixture. Add 20 μM PL for PLP-regeneration to the mixture, baker's yeast OD600 of 100. Add distilled water into the whole-cell mixture containing harvested jhAY cells with OD600 of 5 to reach a total volume of 100 μl. Conduct the reaction at 37°C with settlement. Complete the reaction. Collect each sample from the mixture. Heat the mixture at 95°C for 5 minutes to complete the reaction. Eliminate the cell biomass from the reaction mixture by centrifugation at 13,000 rpm. Dilute the supernatant to an appropriate concentration for analysis to obtain cadaverine [2].
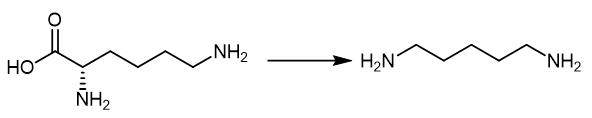
Fig. 4 The synthetic method 3 of 1,5-Diaminopentane.
Add the lysine as substrate at 0.35 M (50 g/L), and use PLP as co-factor at 10 mM. Add the substrate, co-factor and inducer isopropyl-b-D-1-thiogala ctopyranoside (IPTG) at OD600 at 0.5e0.8 (i.e., 3 hours). Keep the mixture culturing the cell for further 24 hours at 37°C with 200 rpm shaking. Apply 2.5e20 mM to determine the PLP effect while 50, 75 and 90 g/L of lysine for enhancing DAP production. Add the IPTG at 3 hours to the mixture. Add the lysine and PLP to the mixture after 12 hours culturing. Analyze the samples every 6 hours to obtain the product [3].
Application
Preparation of a new layered aluminum phosphate
A new layered aluminum phosphate with P:Al ratio 4:3 has been prepared under nonaqueous conditions in the presence of 1,5-diaminopentane and the structure has been determined by single-crystal X-ray diffraction. The compound crystallizes in the monoclinic space group P2(1)/c (Z = 4), with lattice parameters a = 9.801(2), b = 14.837(2), c = 17.815(3) angstrom, beta = 105.65(1)degrees, and V = 2494.7 angstrom3 (R = 0.042 and R(w) = 0.058). The structure consists of AlO4 and PO4 tetrahedra linked to form layers. Two organic cations, diprotonated 1,5-diaminopentane and protonated piperidine derived from cyclization of the starting amine, lie between the inorganic layers and are hydrogen bonded to the layers through NH3+ and NH2+ groups, respectively. The two organic cations are located in two crystallographically distinct eight-membered windows within the layers, the piperidinium cations being associated with circular cavities approximately 6.9 angstrom in diameter, and the 1,5-diaminopentane cations with elliptical cavities (of axes approximately 5.9 and 8.0 angstrom) [4].
The effects of linear diamine chain length in uranium sulfates
The synthesis of uranium sulfates under hydrothermal conditions in the presence of a series of structurally related organic templating agents was investigated. 1,2-ethylenediamine, 1,3-diaminopropane, 1,4-diaminobutane, 1,5-diaminopentane and 1,6-diaminohexane were used under identical reaction conditions to elucidate the effects of variation in linear diamine chain length on the extended structures. As the chain length increases the charge density of the doubly protonated amines decreases. Four new compounds were synthesised and structurally characterised. Two types of inorganic frameworks are observed, evolving as a function of amine chain length. The compounds synthesised using 1,2-ethylenediamine and 1,3-diaminopropane contain [UO2(H2O)(SO4)(4/2)](n)(2n-) chains. The use of 1,4-diaminobutane, 1,5-diaminopentane and 1,6-diaminohexane results in compounds that contain [(UO2)(2)(SO4)(6)](8-) dimers. The extended structures of these compounds are correlated to the charge density of the organic templating agents [5].
As chelating agent
Zirconia is more chemically stable than silica and gamma-alumina, which are commonly used in the preparation of inorganic membranes. However, the hydrolysis of zirconia alkoxides, typical zirconia precursors, proceeds rapidly, leading to difficulties in preparing clear sols. Thus, an attempt was made to reduce the hydrolysis rate of zirconium propoxide by a ligand-exchange reaction with 1,5-diaminopentane (DAP). The availability of water was also reduced by the dropwise addition of glacial acetic acid, which produced an ester by reaction with the solvent, 1-propanol. The addition of DAP was effective in preparing zirconia with a narrow pore-size distribution and a high mesopore volume. DAP also decreased the phase transformation temperature of zirconia and had almost no effect on the micropore structures. The gas permeation properties of the prepared zirconia membranes were fundamentally controlled by the Knudsen diffusion mechanism [6].
References
[1] Guo X, Li M, Li H, et al. Enhanced Cadaverine Production by Engineered Escherichia coli Using Soybean Residue Hydrolysate (SRH) as a Sole Nitrogen Source[J]. Applied Biochemistry and Biotechnology, 2021, 193(2): 533-543.
[2] Han Y H, Kim H J, Choi T R, et al. Improvement of cadaverine production in whole cell system with baker’s yeast for cofactor regeneration[J]. Bioprocess and Biosystems Engineering, 2021, 44(4): 891-899.
[3] Ting W W, Ng I S. Metabolic manipulation through CRISPRi and gene deletion to enhance cadaverine production in Escherichia coli[J]. Journal of bioscience and bioengineering, 2020, 130(6): 553-562.
[4] Chippindale A
- Related articles
- Related Qustion
- Putrescine and cadaverine Aug 7, 2024
Putrescine (butane-1,4-diamine) and cadaverine (pentane-1,5-diamine; 1,5-Diaminopentane) are foul-smelling compounds produced when amino acids decompose in decaying animals.
- 1,5-Diaminopentane: Metabolism and Systems Metabolic Engineering Feb 23, 2024
The metabolism of 1,5-diaminopentane in E. coli and C. glutamicum has been enhanced through genetic modifications, showcasing potential for sustainable production.
1,5-Diaminopentane
462-94-2You may like
1,5-Diaminopentane manufacturers
- 1,5-Diaminopentane
-

- $10.00 / 1KG
- 2025-04-23
- CAS:462-94-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- 1,5-Diaminopentane
-

- $0.00 / 25KG
- 2025-03-21
- CAS:462-94-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- 1,5-Diaminopentane
-

- $10.00 / 1KG
- 2024-10-11
- CAS:462-94-2
- Min. Order: 1KG
- Purity: 99.%
- Supply Ability: 10 ton