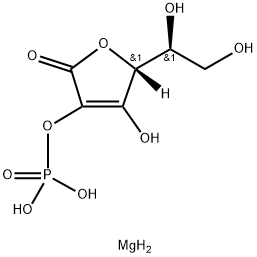
Properties and Determination of Magnesium ascorbyl phosphate
Mar 5,2025
Introduction
Vitamin C (L-ascorbic acid) is a water-soluble vitamin synthesized from D-glucose in the mammalian liver, or in the kidneys in some vertebrates. It can inhibit melanogenesis, promote collagen biosynthesis,and prevent the formation of free radicals in the skin. It has been considered an interesting ingredient of cosmetic skin-care products, but formulating products containing vitamin C is impractical because it is readily soluble in water and is extremely unstable. Therefore, vitamin C is chemically modified by esterification of the hydroxyl group with long-chain organic or inorganic acids. The derivative, magnesium ascorbyl phosphate (MAP, Figure 1), an inorganic water-soluble acid ester, was formulated to overcome the instability of vitamin C [1]. The organic compound that conforms to the formula: C6H8O9P·3/2 Mg. Magnesium ascorbyl phosphate has been widely used in cell and tissue therapy in replace of ascorbate due to its stability and additional cell-biological functionalities. Its stoichiometric composition for its structure was recently measured as (AP)2Mg3, in which Mg2+ is proposed to be complexed to C3-O, C1–O and P–O of AP (APhere refers to ascorbic phosphate).[2-3]

Magnesium Ascorbyl Phosphate Absorption, Distribution, Metabolism, Excretion
Guinea Pigs
Imai et al. [4] reported a study in which male guinea pigs fed a stock diet were fasted for 16 h prior to use, at which time L-Ascorbic Acid (25 mg/animal) or Magnesium Ascorbyl Phosphate (54 mg/animal) dissolved in 1 ml H2O was orally administered. A control group received 1 ml H2O. About 0.6 ml of blood was taken from the heart and the blood ascorbic acid concentration showed rapid elevation within 1 h and began to decline thereafter. No significant differences in the change of the blood Ascorbic acid level between the L-ascorbic acid and Magnesium ascorbyl phosphate groups were measured throughout the experiment.
In Vitro
Kameyama et al.[5] studied the percutaneous absorption of Magnesium ascorbyl phosphate (VC-PMG) in a cream base given the designation VC-PMG by the authors. A 3% VC-PMG cream was spiked with [14C]-labeled VC-PMG and the activity of each cream formulation was determined by liquid scintillation spectrometry.
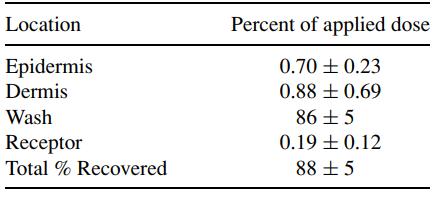
At time 0, approximately 10 mg of each vehicle was pipetted onto dermatomed human cadaver skin that was clamped to flow-through diffusion solution cells with an exposed area of 0.64 cm2. Radiolabel in the epidermis and dermis, in the skin surface wash, and in the receptor phase were measured. The percentage of the applied dose recovered was determined.
Table 1 presents the findings. The percutaneous penetration of the radiolabeled magnesium ascorbyl phosphate was low, ranging from 0.09% to 0.51% of the applied dose. The amount of the radiolabel in the entire skin after topical application of the cream was 0.58%, obtained by the addition of the amount found in the epidermis and dermis.[5-6]
Clinical Treatment
Magnesium ascorbyl phosphate (VC-PMG) cream (10%) was applied twice a day to the skin of 34 patients with ephelides, chloasma, senile freckles, nevus of Ota, or healthy skin. The effectiveness of the lightening of the pigmentation was judged by a color-difference meter. The VC-PMG cream was effective or fairly effective in 19 of 34 patients. Magnesium ascorbyl phosphate cream applied to the healthy skin of 25 patients resulted in 1 effective, 2 fairly effective, 8 slightly effective, 12 not effective, and 2 possible darkenings [5].
Magnesium ascorbyl phosphate functions as an antioxidant in cosmetics and was reported being used in 37 formulations over a wide concentration range (0.001% to 3%). It have been previously reviewed by the CIR Expert Panel and found “to be safe for use as cosmetic ingredients in the present practices of good use.” Magnesium ascorbyl phosphate administration immediately after exposure in hairless mice significantly delayed skin tumor formation and hyperplasia induced by chronic exposure to 2 kJ/m2 of UVB. Many studies have confirmed that magnesium ascorbyl phosphate is safe as used in cosmetic products. [6]
Detection method
Wang et al. [2] had reported a chromogenic reaction between magnesium ascorbyl phosphate and ferric chloride to generate a Brown-Red clathrate, while the Treated magnesium ascorbyl phosphate by phosphatases forms Colorless (BRTC) product with ferric chloride. The BRTC was indicative of phosphatase activity-mediated excision of phosphorous group from magnesium ascorbyl phosphate and utilized to screen phosphatases from bacterial cell lysates. From ten tested strains, BRTC was observed in the cell lysate of Salmonella enterica subsp. enterica serovar Cerro 87. BRTC was again employed to track phosphatase activity of the resuspensions of the ammonium sulfate graded precipitations of the cell lysate. Two phosphatases, PhoN and YcdX, were identified by LC-MS/MS analysis in the protein fraction giving most obvious BRTC phenotype and validated by examination of in vitro activity of the purified proteins.
Ferric chloride mixed with magnesium ascorbyl phosphate can generate the brown-red color substance, which might be the product from ferric ion that simultaneously coordinated with phosphate and C-O groups of AP. Ferric chloride are widely used for phosphorous removal as the ferric ions combine to form ferric phosphate; although the actual reactions for it are not fully understood, we speculate that Fe3+ competes with Mg2+ for coordination with AP in the aqueous solution asin our study excision of the phosphorus group from AP by phosphatase failed to formation of the brown-red substance.
Therefore, the BRTC system is effective to identify magnesium ascorbyl phosphate utilizing phosphatases with a relatively high expression level in microbes.
References
[1] Liao AH, Lu YJ, Hung CR, Yang MY. Efficacy of transdermal magnesium ascorbyl phosphate delivery after ultrasound treatment with microbubbles in gel-type surrounding medium in mice[J]. Mater Sci Eng C Mater Biol Appl. 2016;61:591-598.[2] Wang Y , Hu W , Deng Z ,et al.Rapid identification of magnesium ascorbyl phosphate utilizing phosphatase through a chromogenic change-coupled activity assay[J].Applied Microbiology and Biotechnology, 2021:1-9.
[3] Xu X , Woniczka M , Hecke K V ,et al.Structural study of L-ascorbic acid 2-phosphate magnesium, a raw material in cell and tissue therapy[J]. J Biol Inorg Chem, 2020, 25(6):875-885.
[4] Imai Y , Usui T , Matsuzaki T ,et al.The antiscorbutic activity of L-ascorbic acid phosphate given orally and percutaneously in guinea pigs[J].Jap. J. Pharmacol, 1967, 17: 317-24.
[5] Kameyama K .Inhibitory effect of magnesium L-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo[J].J. Am. Acad. Dermatol., 1996, 34: 29-33.
[6] Elmore AR. Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics [J]. Int J Toxicol. 2005;24 Suppl 2:51-111.
- Related articles
- Related Qustion
Magnesium ascorbyl phosphate
113170-55-1You may like
Magnesium ascorbyl phosphate manufacturers
- Magnesium ascorbyl phosphate
-

- $20.00 / 1kg
- 2025-03-06
- CAS:113170-55-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- MERCARE MAP
-

- $60.00 / 1kg
- 2025-03-06
- CAS:113170-55-1
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 5000
- Magnesium ascorbyl phosphate
-

- $6.00 / 1KG
- 2025-03-06
- CAS:113170-55-1
- Min. Order: 1KG
- Purity: More than 99%
- Supply Ability: 2000KG/Month