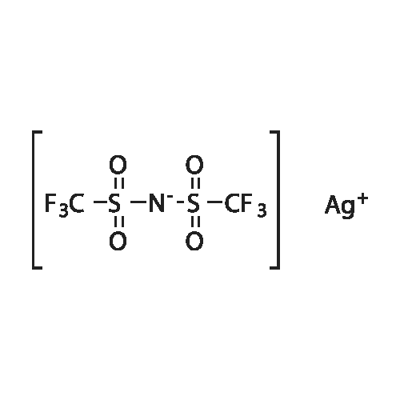
Sliver bis(trifluoromethane sulfonimide) synthesis
- Product Name:Sliver bis(trifluoromethane sulfonimide)
- CAS Number:189114-61-2
- Molecular formula:C2AgF6NO4S2
- Molecular Weight:388
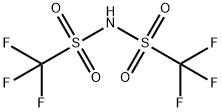
82113-65-3
251 suppliers
$25.00/1 g
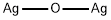
20667-12-3
236 suppliers
$12.00/1g

189114-61-2
108 suppliers
$8.00/250mg
Yield:-
Reaction Conditions:
in isopropyl alcohol for 0.5 h;
Steps:
1 Route B:
A mixture of 0.5 mol N,N,N′,N′-tetramethylethylenediamine (58.1 g) and 0.1 mol of 2-ethylhexyl bromide (19.3 g) was refluxed for 12 h. Unreacted tetramethylethylenediamine was removed under vacuum, and the yield of 2-ethylhexyltetramethylethylenediamine was 100%. Next, a solution of 0.1 mol of silver bis(trifluoromethane)sulfonamide was made by vigorously stirring a suspension of 0.05 mol silver (I) oxide (11.57 g) and 0.1 mol bis(trifluoromethane)sulfonamide (28.1 g) in isopropanol. After 0.5 h of reaction, the solution of silver bis(trifluoromethane)sulfonamide was added dropwise to a solution of 0.1 mol of 2-ethylhexyltetramethylethylenediamine (30.9 g) in isopropanol with continuous stirring. The resulting silver bromide was removed by a sequence of three centrifugations of the reaction mixture and decantation. Isopropanol was then removed by vacuum.
References:
Massachusetts Institute of Technology;Hatton, T. Alan;Brown, Paul;Voskian, Sahag US2020/30716, 2020, A1 Location in patent:Paragraph 0227; 0232; 0238