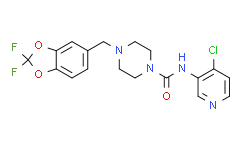
| Description: |
JNJ-42165279 is a FAAH inhibitor with IC50 of 70 ± 8 nM and 313 ± 28 nM for hFAAH and rFAAH, respectively.IC50 value: 70 ± 8 nM (for hFAAH), 313 ± 28 nM (for rFAAH )Target:FAAHJNJ-42165279 covalently inactivates the FAAH enzyme, but is highly selective with regard to other enzymes, ion channels, transporters, and receptors. JNJ-42165279 exhibits high selectivity against a panel of 50 receptors, enzymes, transporters, and ion-channels at 10 μM, at which concentration it does not produce >50% inhibition of binding to any of the targets. Fortunately, JNJ-42165279 also does not inhibit CYPS (1A2, 2C8, 2C9, 2C19, 2D6, 3A4) or hERG when tested at a 10 μM compound concentration. [1]in vivo: JNJ-42165279 exhibits excellent ADME and pharmacodynamic properties as evidenced by its ability to block FAAH in the brain and periphery of rats and thereby cause an elevation of the concentrations of anandamide (AEA), oleoyl ethanolamide (OEA), and palmitoyl ethanolamide (PEA). The compound was also efficacious in the spinal nerve ligation (SNL) model of neuropathic pain. JNJ-42165279 exhibits relatively rapid clearance in the course of rat pharmacokinetic experiments, manifesting as a low AUC and Cmax; however, sufficiently high exposures were obtainable to support preclinical animal models. In a subsequent higher dose (20 mg/kg) oral PK experiment, compound concentrations were determined both in the plasma and brain of rats. [1] |