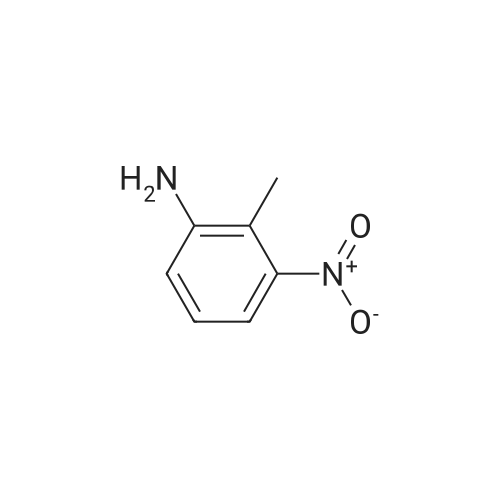
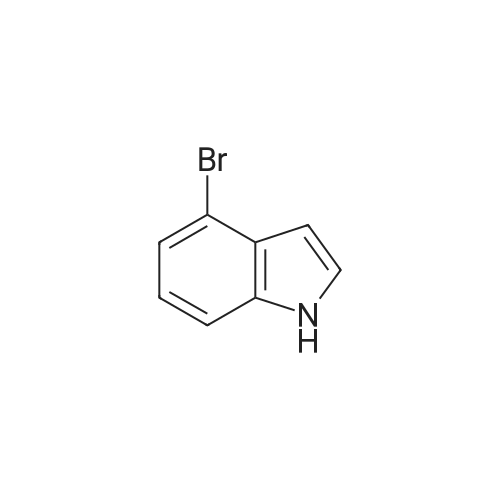
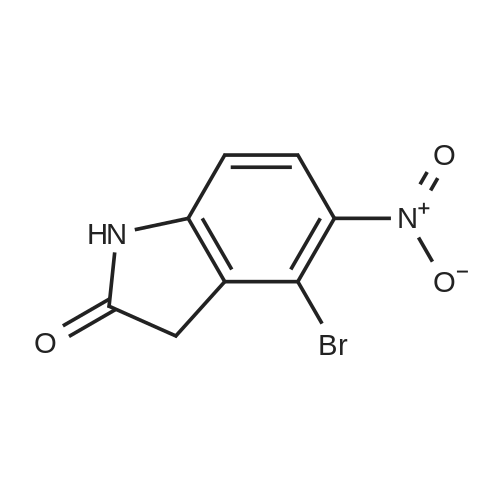
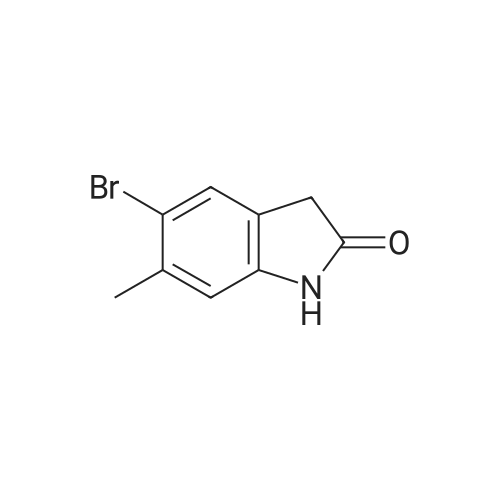
| 74% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate In 1,4-dioxane at 110℃; for 2 h; Sealed tube; Microwave irradiation |
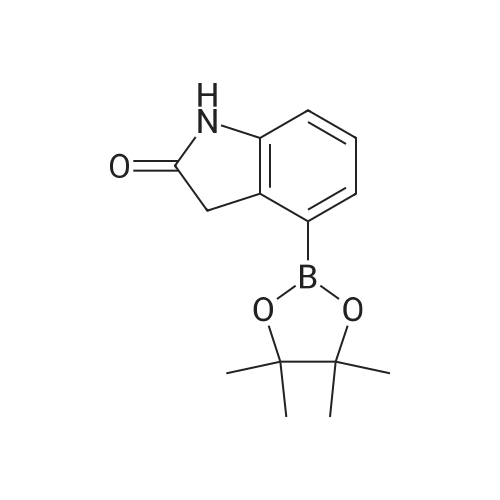
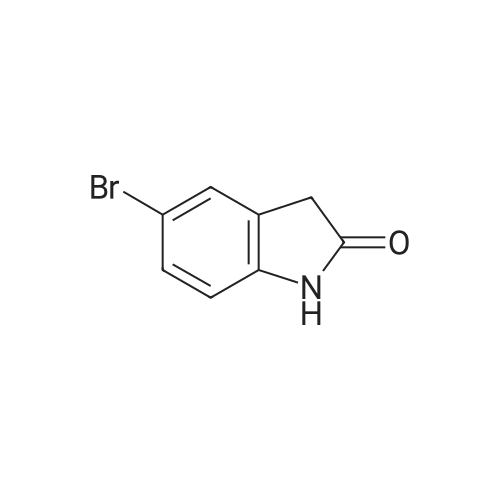
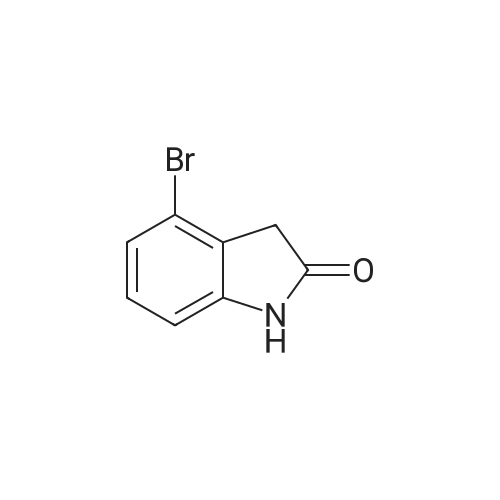
Intermediate 114: 4-(4,4,5,5-Tetramethyl-1 ,2-dioxaborolan-2-yl)indolin-2-one (0793) A mixture of 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (1.903 g, 7.49 mmol, commercially available from, for example, Fluorochem), 4-bromoindolin-2-one (1.038 g, 4.90 mmol, commercially available from, for example, Fluorochem), [1,1'- 7 s(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane [Pd(dppf)Cl2.DCM] (0.601 g, 0.73 mmol) and potassium acetate (1.480 g, 15.08 mmol) in 1,4-dioxane (30 mL) was stirred at 110 °C for 2 h. The mixture was allowed to cool to rt before being filtered through a 10 g celite cartridge. The cartridge was washed through with ethyl acetate (3 x 30 mL) and the combined filtrates were evaporated in vacuoto give a brown liquid which was re-dissolved in DCM (ca. 10 mL), loaded onto a 100 g SNAP silica cartridge and purified by Biotage SP4 semi-automated flash column chromatography eluting with a gradient of 20 to 50percent ethyl acetate in cyclohexane. The required fractions were combined and evaporated in vacuo, this was re-dissolved in DCM (ca. 10 mL), transferred to a tarred vial and the solvent evaporated under a stream of nitrogen. The residue was triturated with ether (5 x 5 mL), decanting away the mother liquor each time, and the residue dried under a stream of nitrogen and in vacuo to give the desired product 4-(4,4,5,5-tetramethyl-l,3,2- dioxaborolan-2-yl)indolin-2-one (941.8 mg, 3.63 mmol, 74 percent yield) as a cream solid. (0794) LCMS (2 min Formic): Rt = 0.93 min, [MH]+ = 260.3. |
| 74.2% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate In 1,4-dioxane at 110℃; for 2 h; |
A mixture of 4,4,4T,4T,5,5,5T,5T-octamethyl-2,2T-bi( 1,3, 2-d ioxaborolane) (1.9025 g, 7.49 mmol), 4-bromoindolin-2-one (1.0383 g, 4.90 mmol), [1,1’-Bis(d iphenylphosphino)ferrocene]dichloropallad ium(II), complex with dichloromethane (0.6005 g,0.734 mmol) and potassium acetate (1.4802 g, 15.08 mmol) in 1,4-Dioxane (30 mL) was stirred at110 °C for 2 hr. The mixture was allowed to cool to room temperature before being filtered througha lOg celite cartridge. The cartridge was washed through with ethyl acetate (3 x 30 mL) and thecombined filtrates were evaporated in vacuo to give to give a brown liquid, which was redissolved indichloromethane (ca. 10 mL), loaded onto a bOg SNAP silica cartridge and purified by Biotage 5P4semi-automated flash column chromatography eluting with a gradient of 20 to 50percent ethyl acetate incyclohexane. The required fractions were combined and evaporated in vacuo, the residue (which was on the verge of crystallisation) was re-dissolved in dichloromethane (ca. 10 mL), transferred to a tared vial, the solvent evaporated under a stream of nitrogen. The residue was triturated with ether (5 x 5 mL), decanting away the mother liquor each time, and the residue dried under a stream of nitrogen and in vacuoto give the desired product as a cream solid (941.8 mg, 3.63 mmol, 74.2 percent yield)LCMS (2 mm Formic): Rt = 0.93 mi [MH]+ = 260 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping