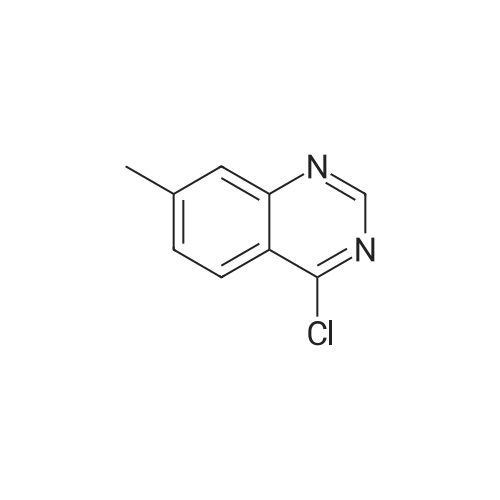
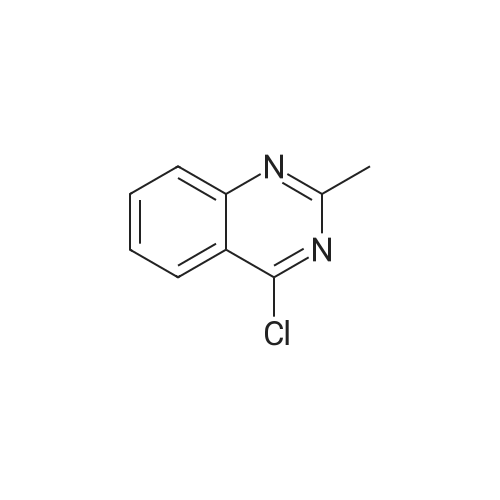
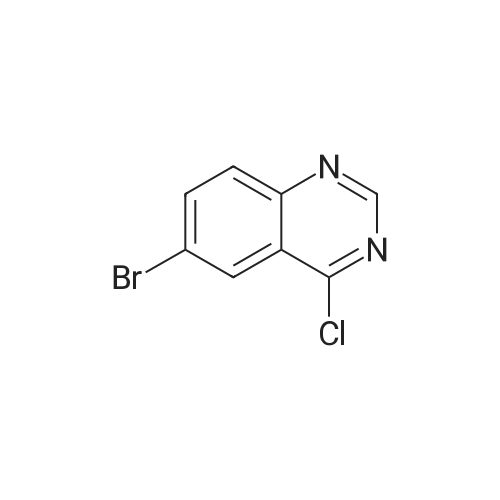
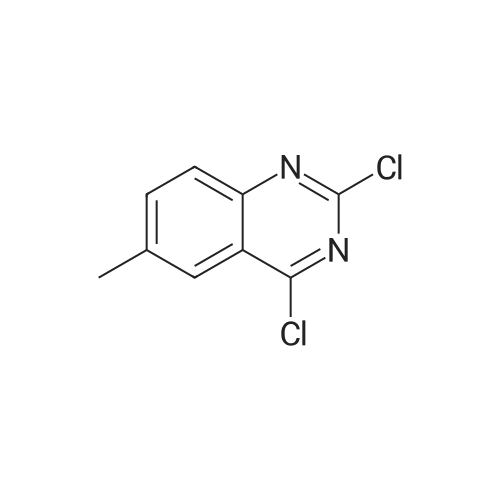
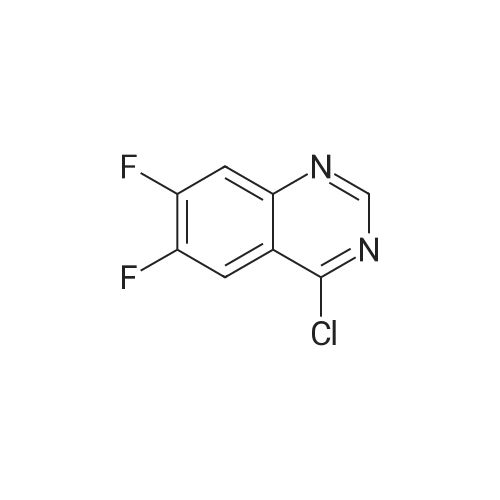
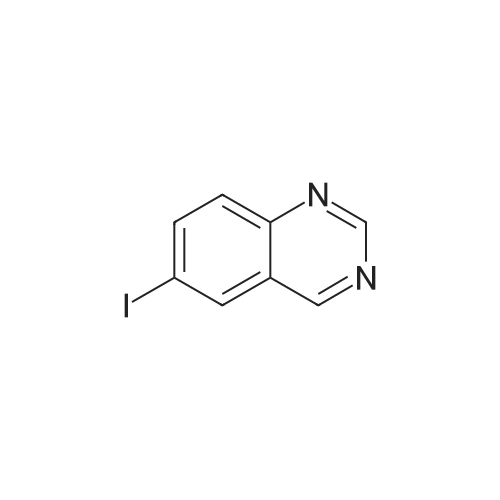
| 77% |
With thionyl chloride; In N,N-dimethyl-formamide;Reflux; |
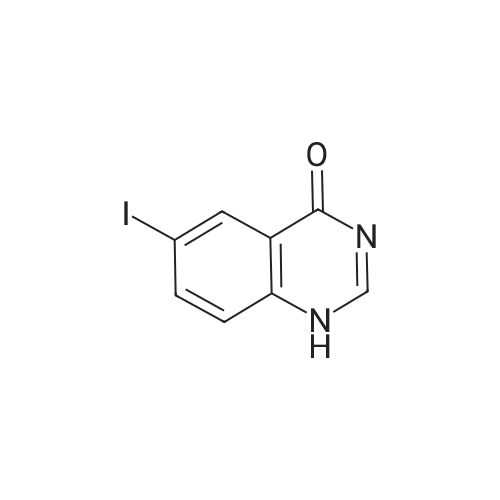
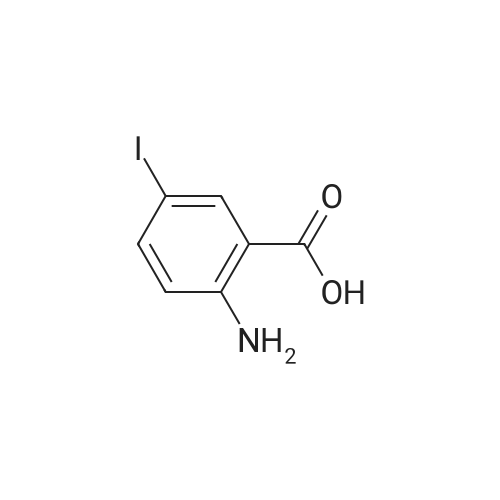
6-iodo-4(1H)-quinazolinone (10 g, 37 mmol)was weighed into a 250 mL flask. Thionyl chloride (100 mL, 1.4 mmol) and DMF (0.5mL, 6.5 mmol) were added to give a grey suspension. The mixture was heated to reflux.Heating was continued for 6 h and then the mixture was cooled on ice bath for 1 h. A yellow solid precipitated and was collected by filtration to afford 8.6 g (77%) of the title compound. |
| 53% |
With trichlorophosphate; |
6-Iodoquinazolin-4(3H)-one (1Og, 37 mmol) was refluxed in POCI3 (100 mL) overnight. Then POCI3 was removed in vacuo. The residue was dissolved in CH2Cl2 (500 mL ). The organic phase was washed with water (100 mL) and dried (MgSO4). Then CH2Cl2 was removed in vacuo and 1404-174 was obtained (5.7 g, 53%): LC- MS: 291 [M+l]+, 1H NMR (CDCl3): δ 7.81 (d, J= 9.0 Hz, 1 H), 8.21 (dd, Ji = 9.0 Hz, J2 = 1.8 Hz, I H), 8.65 (d, J= 1.8 Hz, 1 H), 9.06 (s, 1 H). |
|
With tributyl-amine; trichlorophosphate; In toluene; at 20 - 90℃;Heating / reflux; |
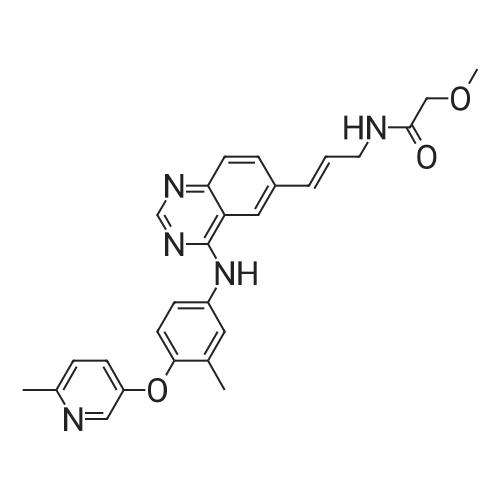
Example 1; Preparation of GW572016F; STAGE 1; A stirred suspension of 3H-6-iodoquinazolin-4-one (compound A) in toluene (5 vols) was treated with tri-n-butylamine (1.2 eq.) at 20 to 25C, then heated to 900C. Phosphorous oxychloride (1.1 eq) was added, the reaction mixture was then heated to reflux. The reaction mixture was cooled to 500C and toluene (5vols) added. Compound C (1.03 eq.) was added as a solid, the slurry was warmed back to 900C and stirred for 1 hour. The slurry was transferred to a second vessel; the first vessel was rinsed with toluene (2vol) and combined with the reaction mixture. The reaction mixture was cooled to 700C and 1.0 M aqueous sodium hydroxide solution (16 vols) added dropwise over 1 hour to the stirred slurry maintaining the contents temperature between 68-72C. The mixture was stirred at 65-700C for 1 hour and then cooled to 200C over 1 hour. The suspension was stirred at 200C for 2 hours, the product collected by filtration, and washed successively with water (3 x 5 vols) and ethanol (IMS, 2 x 5 vols), then dried in vacuo at 50-600C. <n="25"/>Volumes are quoted with respect of the quantity of Compound A used. Percent yield range observed: 90 to 95% as white or yellow crystals. |
|
With tributyl-amine; trichlorophosphate; In toluene; at 20 - 90℃;Heating / reflux; |
A stirred suspension of 3H-6-iodoquinazolin-4-one (compound A) in toluene (5 vols) was treated with tri-n-butylamine (1.2 eq.) at 20 to 25C, then heated to 900C. Phosphorous oxychloride (1.1eq) was added, the reaction mixture was then heated to reflux. The reaction mixture was cooled to 500C and toluene (5vols) added. Compound C (1.03 eq.) was added as a solid, the slurry was warmed back to 900C and stirred for 1 hour. The slurry was transferred to a second vessel; the first vessel was rinsed with toluene (2vol) and combined with the reaction mixture. The reaction mixture was cooled to 7O0C and 1.0 M aqueous sodium hydroxide solution (16 vols) added dropwise over 1 hour to the stirred slurry maintaining the contents temperature between 68-72C. The mixture was stirred at 65-700C for 1 hour and then cooled to 200C over 1 hour. The suspension was stirred at 200C for 2 hours, the product collected by filtration, and washed successively with water (3 x 5 vols) and ethanol (IMS, 2 x 5 vols), then dried in vacuo at 50-600C. Volumes are quoted with respect of the quantity of Compound A used. Percent yield range observed: 90 to 95% as white or yellow crystals. |
|
With tributyl-amine; trichlorophosphate; In toluene; at 70 - 80℃; for 2h;Heating / reflux; |
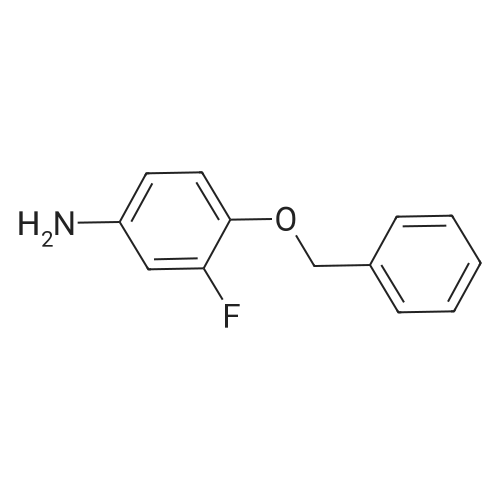
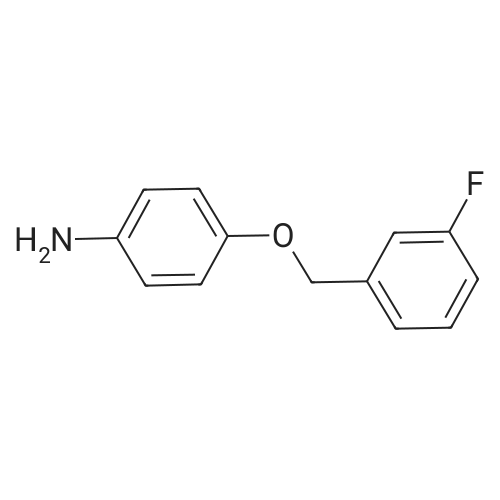
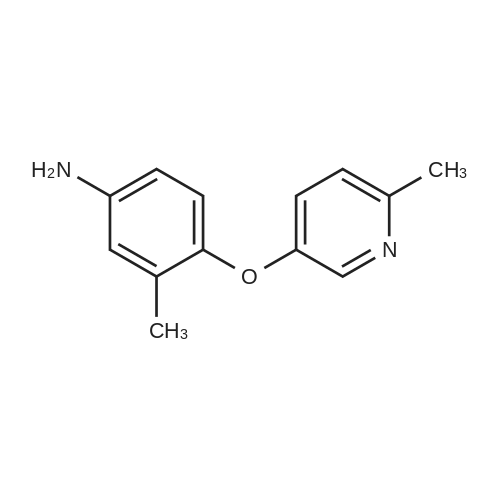
A stirred suspension of 3W-6-iodoquinazolin-4-one in toluene (5 vols) is treated with tri-n-butylamine (1.2 equiv.), and then heated to 70-800C. Phosphorous oxychloride (1.1 equiv.) is added and the reaction mixture is then heated to reflux and stirred at this temperature for at least 2 hours. The reaction mixture is then cooled to 55C and toluene (5vol) added followed by 3-chloro-4-[(3-fluorophenyl)rnethyl]oxy}aniline (1.03 equiv.). The reaction mixture is then warmed to 70-900C and stirred for at least 2 hours. The resultant slurry is transferred to a second vessel. The temperature is adjusted to 70-750C and 8 molar aqueous sodium hydroxide solution (2 vols) added over 1 hour, followed by water (6vol.) maintaining the contents at 70-850C. The mixture is stirred at 70-850C for ca. 1 hour and then cooled to 20-250C. The suspension is stirred for ca. 2 hours and the product collected by filtration, and washed successively with water, 0.1 molar aqueous sodium hydroxide, water, and IMS, then dried in vacuo. EPO <DP n="48"/> |
|
With thionyl chloride; In N,N-dimethyl-formamide;Reflux; |
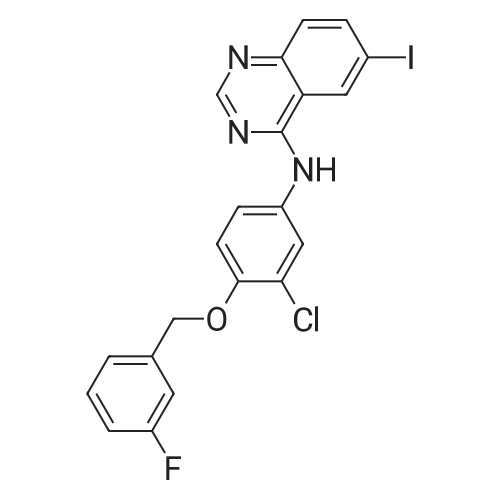
Example 1 Preparation of N-(4-(3-fluorobenzyloxy)-3-chlorophenyl)-6-iodine-quinazolin-4-amine 6-Iodine-3H-quinazolin-4-ketone (100 g) was added into a 2000 mL flask, dissolved in a mixed solvent of thionyl chloride (1000 mL) and N,N-dimethylformamide (20 mL), heated to reflux until the reaction solution is clear and transparent. After thionyl chloride was removed, anhydrous toluene was added to the residues and removed under reduced pressure, and the process of the adding and removing of toluene was repeated again to removed the remained thionyl chloride residues. The intermediate was dissolved in isopropyl alcohol (2000 mL), 3-chloro-4-(3-fluoro-benzyloxy)-aniline hydrochloride was added, and anhydrous K2CO3 (150 g) was added with mechanical stirring before the mixture was heated to reflux over night. The reaction solution was cooled to room temperature overnight, the precipitation was filtered and washed with water for multi-times until the pH of washing solution reached neutral. After drying under vacuum, 95 g of the title product was collected in a pale white solid. m/z M+1+: 506 |
|
With thionyl chloride; for 2h;Reflux; |
Example 1 Synthesis of CD 8/CD 19 inhibitors SNX-2-1-165 was synthesized according to the scheme shown in Figure 2. All other compounds were synthesized using similar procedures. |
|
With tributyl-amine; trichlorophosphate; In toluene; at 20℃;Heating / reflux; |
A stirred suspension of 3H-6-iodoquinazolin-4-one (compound A) in toluene (5 vols) was treated with tri-n-butylamine (1.2 eq. ) at 20 to 25C, then heated to 90C. Phosphorous oxychloride (1.1eq) was added, the reaction mixture was then heated to reflux. The reaction mixture was cooled to 50C and toluene (5vols) added. Compound C (1.03 eq. ) was added as a solid, the slurry was warmed back to 90C and stirred for 1 hour. The slurry was transferred to a second vessel; the first vessel was rinsed with toluene (2vol) and combined with the reaction mixture. The reaction mixture was cooled to 70C and 1.0 M aqueous sodium hydroxide solution (16 vols) added dropwise over 1 hour to the stirred slurry maintaining the contents temperature between 68-72C. The mixture was stirred at 65-70C for 1 hour and then cooled to 20C over 1 hour. The suspension was stirred at 20C for 2 hours, the product collected by filtration, and washed successively with water (3 x 5 vols) and ethanol (IMS, 2 x 5 vols), then dried in vacuo at 50-60C. Volumes are quoted with respect of the quantity of Compound A used. Percent yield range observed: 90 to 95% as white or yellow crystals. |
|
With tributyl-amine; trichlorophosphate; In toluene; at 20℃;Heating / reflux; |
A stirred suspension of 3H-6-iodoquinazolin-4-one (compound A) in toluene (5 vols) was treated with tri-n-butylamine (1.2 eq. ) at 20 to 25C, then heated to 90C. Phosphorous oxychloride (1.1 eq) was added, the reaction mixture was then heated to reflux. |
|
With P,P-dichlorophenylphosphine oxide; at 150℃; for 1h; |
A mixture of 6-iodoquinazolin-4 (3H)-one (Intermediate 11,100 mg, 0.37 mmol) and phenylphosphonic dichloride (1.5 ML) was heated at 150 C for one h. After cooling the reaction mixture in an ice bath, isopropyl ether was added. The resultant crystalline precipitate was collected by filtration, and was then stirred with saturated aqueous sodium bicarbonate solution. The solution was extracted with three portions of ethyl acetate, and the combined extracts were dried (magnesium sulfate) and concentrated under reduced pressure to give the crude product. Purification by preparative thin layer chromatography (silica gel, 5% and 10% ethyl acetate/hexanes) afforded the desired product. LC/MS 291 (M+1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping