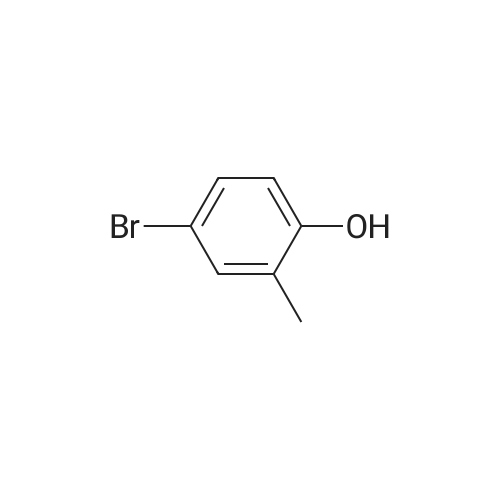
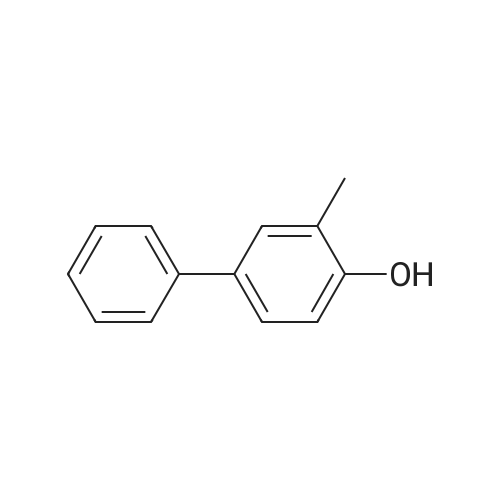
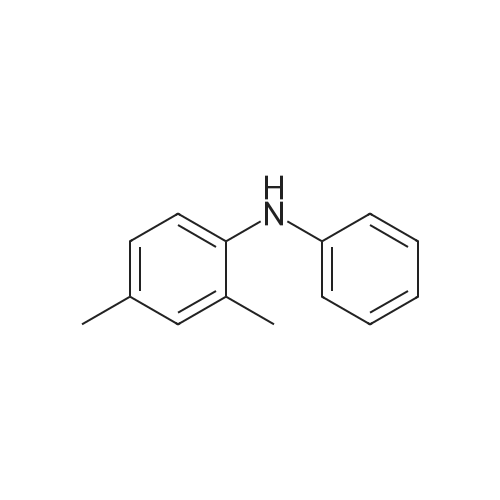
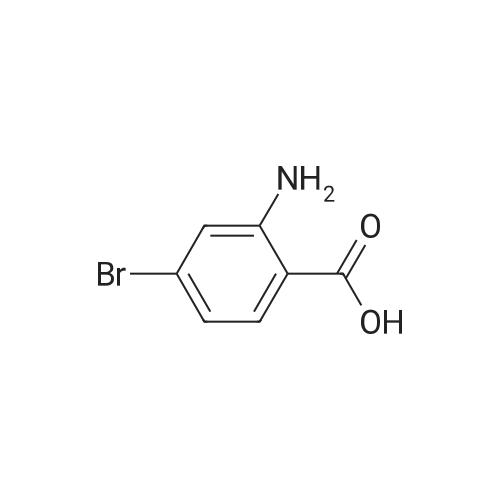
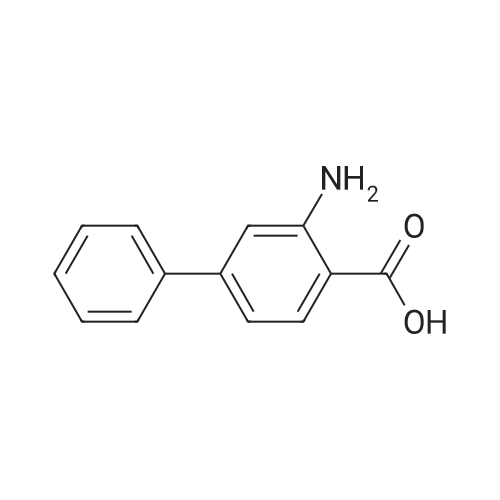
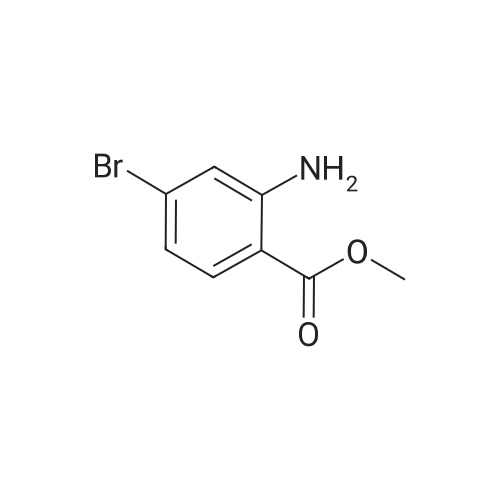
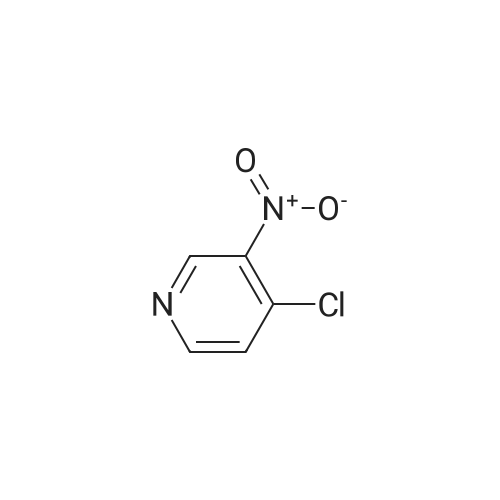
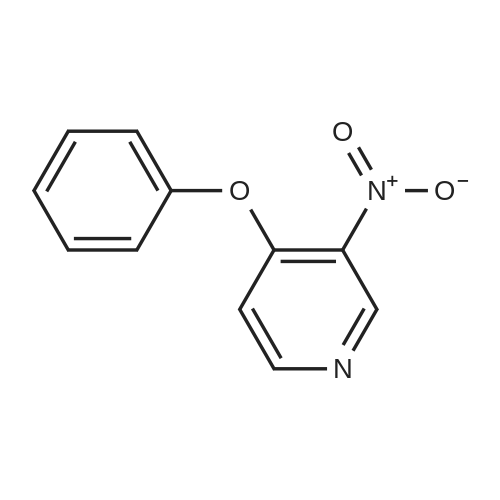
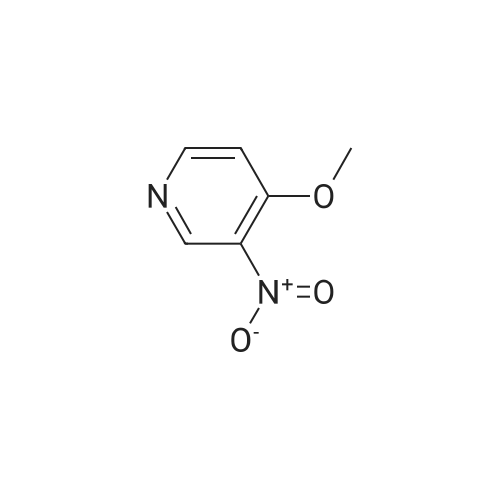
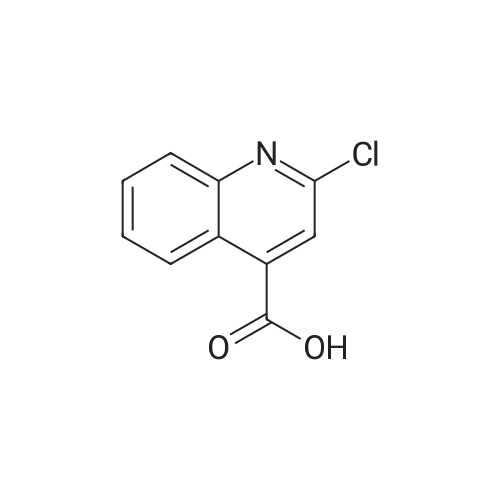
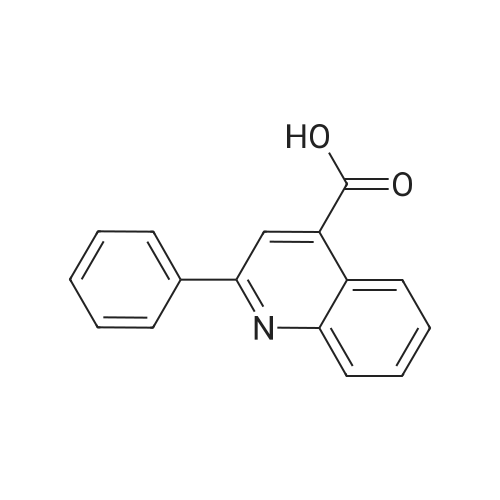
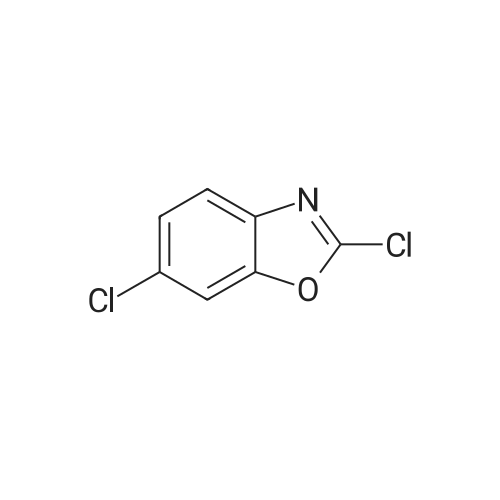
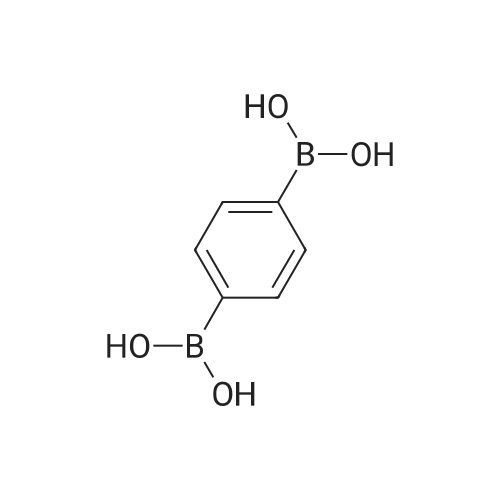
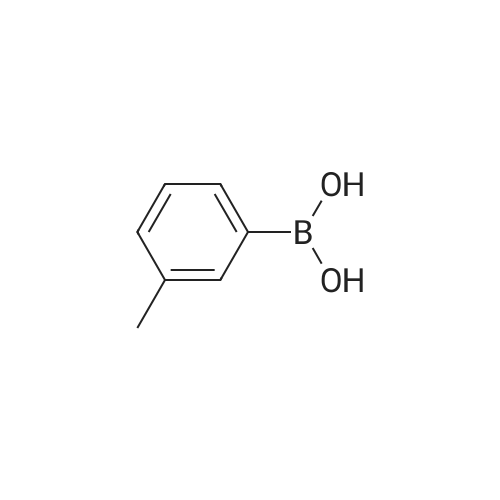
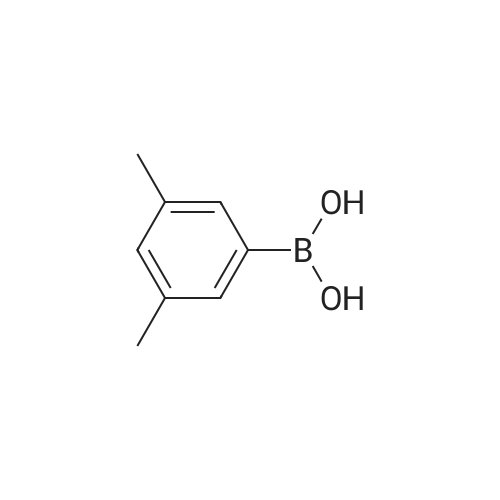
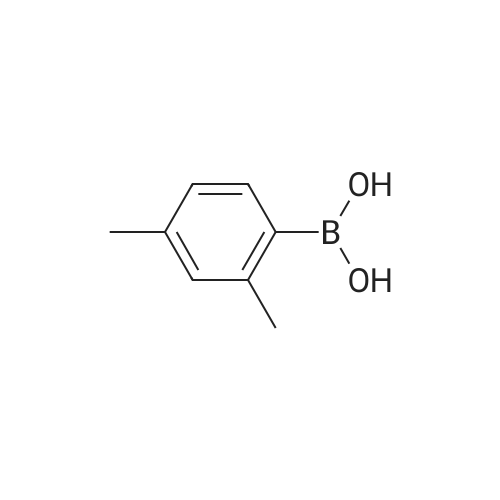
Synthesis and biological evaluation of structurally diverse 6-aryl-3-aroyl-indole analogues as inhibitors of tubulin polymerization
Wen Ren
;
Yuling Deng
;
Jacob D. Ward
, et al.
Eur. J. Med. Chem.,2024,263,115794.
DOI:
10.1016/j.ejmech.2023.115794
More
Abstract: The synthesis and evaluation of small-molecule inhibitors of tubulin polymerization remains a promising approach for the development of new therapeutic agents for cancer treatment. The natural products colchicine and combretastatin A-4 (CA4) inspired significant drug discovery campaigns targeting the colchicine site located on the beta-subunit of the tubulin heterodimer, but so far these efforts have not yielded an approved drug for cancer treatment in human patients. Interest in the colchicine site was enhanced by the discovery that a subset of colchicine site agents demonstrated dual functionality as both potent antiproliferative agents and effective vascular disrupting agents (VDAs). Our previous studies led to the discovery and development of a 2-aryl-3-aroyl-indole analogue (OXi8006) that inhibited tubulin polymerization and demonstrated low nM IC50 values against a variety of human cancer cell lines. A water-soluble phosphate prodrug salt (OXi8007), synthesized from OXi8006, displayed promising vascular disrupting activity in mouse models of cancer. To further extend structure-activity relationship correlations, a series of 6-aryl-3-aroyl-indole analogues was synthesized and evaluated for their inhibition of tubulin polymerization and cytotoxicity against human cancer cell lines. Several structurally diverse molecules in this small library were strong inhibitors of tubulin polymerization and of MCF-7 and MDA-MB-231 human breast cancer cells. One of the most promising analogues (KGP591) caused significant G2/M arrest of MDA-MB-231 cells, disrupted microtubule structure and cell morphology in MDA-MB-231 cells, and demonstrated significant inhibition of MDA-MB-231 cell migration in a wound healing (scratch) assay. A phosphate prodrug salt, KGP618, synthesized from its parent phenolic precursor, KGP591, demonstrated significant reduction in bioluminescence signal when evaluated in vivo against an orthotopic model of kidney cancer (RENCA-luc) in BALB/c mice, indicative of VDA efficacy. The most active compounds from this series offer promise as anticancer therapeutic agents.
Keywords:
Inhibitors of tubulin polymerization ;
Vascular disrupting agents ;
Indole synthesis ;
Molecular docking ;
Antiproliferative agents ;
Inhibitors of cell migration
Purchased from AmBeed:
128796-39-4 ;
10365-98-7 ;
98-80-6 ;
98437-24-2 ;
5720-05-8 ;
64-86-8 ;
13331-27-6 ;
206551-43-1 ;
63139-21-9 ;
622864-48-6 ;
5720-07-0 ;
87199-18-6 ;
30418-59-8 ;
4521-61-3 ;
4521-61-3 ;
87199-18-6 ;
64-86-8 ;
64-86-8 ;
128796-39-4 ;
5720-05-8 ;
64-86-8
...More

Sustainable methodologies for synthesis of small organic molecules using micellar catalysis
Deborah Sam Ogulu
;
University of Louisville,2024,4274.
More
Abstract: Organic synthesis is a critical process in the creation of small molecule pharmaceuticals and agrochemicals. However, most methods for synthesizing these small molecules rely on toxic organic solvents as the reaction medium which account for approximately 80% of pharmaceutical waste. Moreover, many catalytic reactions require expensive endangered precious metals like palladium and costly metals. This dissertation presents research that aims to develop sustainable, eco-friendly reaction conditions to address these issues. Chapter 1 provides an overview of green and sustainable chemistry and chemistry in water. It explains what sustainability entails and the drive towards greener synthetic methods. Also included is the introduction to the concept of chemistry in water, the different types of roles of water in chemistry, and the development of micellar catalysis – including its evolution, applications, current challenges, and future directions. Chapter 2 discusses the development of a ligand-free bimetallic nanocatalyst for the hydrogenation of unsaturated enones. This ligand-free nanocatalyst was prepared from nickel and ppm loading of palladium and was stabilized by harnessing the structural features of the amphiphile, PS-750-M. The physical properties of the nanoparticles were evaluated and thoroughly characterized using different analytical techniques like HRTEM, XPS, and TGA. Chapter 3 describes the application of a copper catalyst in the hydroboration of unsymmetrical internal alkynes with high regioselectivity under aqueous micellar conditions. The methodology was amenable to internal alkynes with diverse functional groups and provides a unique route to access β selective alkenyl boronates. Chapter 4 showcases the development of a protocol towards coupling of aryl boronic acids and primary amines under aqueous micellar conditions using an inexpensive nickel catalyst and oxygen balloon. The developed methodology provides another way to access amines under more sustainable reaction conditions. Chapter 5 describes the use of ppm palladium and copper catalysts immobilized on silica for the catalytic dehydration of amides to nitriles. The protocol employs acetonitrile as the additive and the reaction is performed using aqueous PS-750-M as the reaction medium.
Purchased from AmBeed:
98-80-6 ;
1120-90-7 ;
591-50-4

Enhanced dynamic covalent chemistry for the controlled release of small molecules and biologics from a nanofibrous peptide hydrogel platform
Brett H. Pogostin
;
Samuel X. Wu
;
Michael J. Swierczynski
, et al.
bioRxiv,2024:2024.05.21.595134.
DOI:
10.1101/2024.05.21.595134
More
Abstract: Maintaining safe and potent pharmaceutical drug levels is often challenging. Multidomain peptides (MDPs) assemble into supramolecular hydrogels with a well-defined, highly porous nanostructure that makes them attractive for drug delivery, yet their ability to extend release is typically limited by rapid drug diffusion. To overcome this challenge, we developed self-assembling boronate ester release (SABER) MDPs capable of engaging in dynamic covalent bonding with payloads containing boronic acids (BAs). As examples, we demonstrate that SABER hydrogels can prolong the release of five BA-containing small-molecule drugs as well as BA-modified insulin and antibodies. Pharmacokinetic studies revealed that SABER hydrogels extended the therapeutic effect of ganfeborole from days to weeks, preventing Mycobacterium tuberculosis growth better than repeated oral administration in an infection model. Similarly, SABER hydrogels extended insulin activity, maintaining normoglycemia for six days in diabetic mice after a single injection. These results suggest that SABER hydrogels present broad potential for clinical translation.
Purchased from AmBeed:
98-80-6 ;
1072833-77-2 ;
174671-46-6 ;
179324-69-7

Synthetic and mechanistic strategies to achieve unconventional site-selectivity in cross-couplings of dihalo-heteroarenes
Norman, Jacob Patrick
;
Montana State University,2024.
DOI:
/
More
Abstract: Pd-catalyzed cross-couplings rank among the most powerful methods for constructing substituted biaryls, polyaryls, and heteroarenes. Frequently, di- or polyhalogenated (hetero)arenes are employed as starting materials in cross-couplings to access products with increased structural complexity via multiple cross-coupling or substitution steps. N-heteroarenes bearing multiple reactive handles—such as halides, are of particular interest as starting materials since their crosscoupled products can be medicinally relevant. Non-symmetrical dihalogenated N-heteroarenes typically exhibit a site-selectivity bias for C—X bonds which are adjacent to at least one heteroatom in Pd-catalyzed cross-couplings. However, some Pd catalysts—particularly those with hindered ligands, promote atypical selectivity at distal C—X bonds of 2,X-dichloropyridines and related heterocycles during the selectivity-determining oxidative addition step. This dissertation explores the mechanistic origins of these ligand trends and emphasizes the critical importance of Pd’s ligation state—either mono (PdL) or bis (PdL2), in controlling the site of oxidative addition. Ligation state is also relevant when selecting for the products of mono- vs difunctionalization in cross-couplings of dihalogenated substrates, since bisligated 14 e- Pd dissociates quickly from the monofunctionalized intermediate after an initial cross-coupling cycle, whereas monoligated 12 e- Pd is slow to dissociate and may "ring-walk" to the remaining reactive site(s). Additionally, this dissertation explores alternative methods to access minor regioisomers in cross-couplings of dichloro-azines. One approach involves ligand-free conditions where atypical site-selectivity at dichloropyridines and dichloropyrimidines arises from a change in Pd’s speciation from mono- to multinuclearity. Another approach employs a thiolation/Liebeskind-Srogl arylation sequence to achieve site-selectivity which is orthogonal to that of Suzuki-Miyaura couplings.
Purchased from AmBeed:
3934-20-1 ;
98-80-6 ;
326477-70-7 ;
49844-90-8 ;
68986-76-5

Dioxin-Linked Covalent Organic Framework-Supported Palladium Complex for Rapid Room-Temperature Suzuki-Miyaura Coupling Reaction
Campbell, Allea
;
Alsudairy, Ziad
;
Dun, Chaochao
, et al.
Crystals,2023,13(8):1268.
DOI:
10.3390/cryst13081268
More
Abstract: Covalent organic framework (COF)-supported palladium catalysts have garnered enormous attention for cross-coupling reactions. However, the limited linkage types in COF hosts and their suboptimal catalytic performance have hindered their widespread implementation. Herein, we present the first study immobilizing palladium acetate onto a dioxin-linked COF (Pd/COF-318) through a facile solution impregnation approach. By virtue of its permanent porosity, accessible Pd sites arranged in periodic skeletons, and framework robustness, the resultant Pd/COF-318 exhibits exceptionally high activity and broad substrate scope for the Suzuki-Miyaura coupling reaction between aryl bromides and arylboronic acids at room temperature within an hour, rendering it among the most effective Pd/COF catalysts for Suzuki-Miyaura coupling reactions to date. Moreover, Pd/COF-318 demonstrates excellent recyclability, retaining high activity over five cycles without significant deactivation. The leaching test confirms the heterogeneity of the catalyst. This work uncovers the vast potential of dioxin-linked COFs as catalyst supports for highly active, selective, and durable organometallic catalysis.
Keywords:
covalent organic framework (COF) ;
dioxin-linked COF ;
Pd(II) immobilization ;
Suzuki-Miyaura coupling
Purchased from AmBeed:
24067-17-2 ;
98-80-6 ;
3375-31-3 ;
71597-85-8 ;
4877-80-9 ;
99768-12-4

Synthesis and Characterization of 5-(2-Fluoro-4-[11C]methoxyphenyl)-2,2-dimethyl-3,4-dihydro-2H-pyrano[2,3-b]pyridine-7-carboxamide as a PET Imaging Ligand for Metabotropic Glutamate Receptor 2
Yuan, Gengyang
;
Dhaynaut, Maeva
;
Lan, Yu
, et al.
J. Med. Chem.,2022,65(3):2593-2609.
DOI:
10.1021/acs.jmedchem.1c02004
PubMed ID:
35089713
More
Abstract: Metabotropic glutamate receptor 2 (mGluR2) is a therapeutic target for several neuropsychiatric disorders. An mGluR2 function in etiology could be unveiled by positron emission tomography (PET). In this regard, 5-(2-fluoro-4-[11C]methoxyphenyl)-2,2-dimethyl-3,4-dihydro-2H-pyrano[2,3-b]pyridine-7-carboxamide ([11C]13, [11C]mG2N001), a potent negative allosteric modulator (NAM), was developed to support this endeavor. [11C]13 was synthesized via the O-[11C]methylation of phenol 24 with a high molar activity of 212 ± 76 GBq/μmol (n = 5) and excellent radiochemical purity (>99%). PET imaging of [11C]13 in rats demonstrated its superior brain heterogeneity and reduced accumulation with pretreatment of mGluR2 NAMs, VU6001966 (9) and MNI-137 (26), the extent of which revealed a time-dependent drug effect of the blocking agents. In a nonhuman primate, [11C]13 selectively accumulated in mGluR2-rich regions and resulted in high-contrast brain images. Therefore, [11C]13 is a potential candidate for translational PET imaging of the mGluR2 function.
Purchased from AmBeed:
16289-54-6 ;
5521-55-1 ;
22047-25-2 ;
98-80-6 ;
40155-47-3 ;
5720-05-8 ;
879-65-2 ;
98-96-4 ;
31519-62-7 ;
23688-89-3 ;
23611-75-8 ;
33332-25-1 ;
20737-42-2 ;
61442-38-4 ;
17933-03-8 ;
50681-25-9 ;
13924-99-7 ;
40155-43-9 ;
166744-78-1 ;
36070-80-1 ;
4595-61-3 ;
118853-60-4 ;
41110-28-5 ;
40155-42-8 ;
937669-80-2 ;
31462-59-6 ;
16419-60-6 ;
5424-01-1 ;
59-67-6 ;
34604-60-9 ;
27398-39-6 ;
1196151-53-7 ;
19847-12-2 ;
13965-03-2 ;
876161-05-6 ;
27825-21-4 ;
2164-61-6 ;
4604-72-2 ;
98-97-5 ;
24005-61-6 ;
5521-61-9 ;
2516-34-9 ;
2719-27-9 ;
123-90-0 ;
6761-50-8 ;
625-43-4 ;
872-64-0 ;
1309866-36-1 ;
36932-49-7 ;
1528085-68-8 ;
1195533-51-7 ;
13534-79-7
...More
-2,2-dimethyl-3,4-dihydro-2H-pyrano[2,3-b]pyridine-7-carboxamide as a PET Imaging Ligand for Metabotropic Glutamate Recept.png)

Near-infrared light photocatalysis enabled by a ruthenium complex-integrated metal-organic framework via two-photon absorption
Tang, Jian-Hong
;
Han, Guanqun
;
Li, Guodong
, et al.
IScience,2022,25(4):104064.
DOI:
10.1016/j.isci.2022.104064
PubMed ID:
35355522
More
Abstract: Photocatalysis under UV/visible light irradiation has emerged as one of the green methodologies for solar energy utilization and organic synthesis. These photocatalytic processes are typically initiated by one-photon-absorbing metal complexes or organic dyes. Nevertheless, the intrinsic restrictions of UV/visible light irradiation, such as shallow penetration in reaction solutions, competing absorption by substrates, and limited coverage of the solar spectrum, call for the development of innovative photocatalysts functioning under longer wavelength irradiation Herein, we report a ruthenium complex containing a metal-organic framework, MOF-Ru1, which can drive diverse organic reactions under 740 nm light irradiation following the two-photon absorption (TPA) process. Various organic transformations such as energy transfer, reductive, oxidative, and redox neutral reactions were realized using this heterogeneous hybrid photocatalyst. Overall, MOF-Ru1 represents an intriguing TPA photocatalyst active under near-IR light irradiation, paving a way for the efficient utilization of low-energy light and convenient photocatalyst recycling because of phase separation
Purchased from AmBeed:
98-80-6

Structure activity relationship of pyrazinoic acid analogs as potential antimycobacterial agents
Hegde, Pooja V.
;
Aragaw, Wassihun W.
;
Cole, Malcolm S.
, et al.
Bioorgan. Med. Chem.,2022,74,117046.
DOI:
10.1016/j.bmc.2022.117046
PubMed ID:
36228522
More
Abstract: Tuberculosis (TB) remains a leading cause of infectious disease-related mortality and morbidity. Pyrazinamide (PZA) is a critical component of the first-line TB treatment regimen because of its sterilizing activity against non-replicating Mycobacterium tuberculosis (Mtb), but its mechanism of action has remained enigmatic. PZA is a prodrug converted by pyrazinamidase encoded by pncA within Mtb to the active moiety, pyrazinoic acid (POA) and PZA resistance is caused by loss-of-function mutations to pyrazinamidase. We have recently shown that POA induces targeted protein degradation of the enzyme PanD, a crucial component of the CoA biosynthetic pathway essential in Mtb. Based on the newly identified mechanism of action of POA, along with the crystal structure of PanD bound to POA, we designed several POA analogs using structure for interpretation to improve potency and overcome PZA resistance. We prepared and tested ring and carboxylic acid bioisosteres as well as 3, 5, 6 substitutions on the ring to study the structure activity relationships of the POA scaffold. All the analogs were evaluated for their whole cell antimycobacterial activity, and a few representative mols. were evaluated for their binding affinity, towards PanD, through isothermal titration calorimetry. We report that analogs with ring and carboxylic acid bioisosteres did not significantly enhance the antimicrobial activity, whereas the alkylamino-group substitutions at the 3 and 5 position of POA were found to be up to 5 to 10-fold more potent than POA. Further development and mechanistic anal. of these analogs may lead to a next generation POA analog for treating TB.
Keywords:
Tuberculosis ;
Pyrazinoic acid ;
pyrazinamide
Purchased from AmBeed:
16289-54-6 ;
5521-55-1 ;
22047-25-2 ;
98-80-6 ;
40155-47-3 ;
5720-05-8 ;
879-65-2 ;
98-96-4 ;
31519-62-7 ;
23688-89-3 ;
23611-75-8 ;
33332-25-1 ;
20737-42-2 ;
61442-38-4 ;
17933-03-8 ;
50681-25-9 ;
13924-99-7 ;
40155-43-9 ;
36070-80-1 ;
4595-61-3 ;
118853-60-4 ;
41110-28-5 ;
40155-42-8 ;
937669-80-2 ;
98-98-6 ;
31462-59-6 ;
16419-60-6 ;
5424-01-1 ;
59-67-6 ;
34604-60-9 ;
27398-39-6 ;
1196151-53-7 ;
19847-12-2 ;
13965-03-2 ;
876161-05-6 ;
27825-21-4 ;
2164-61-6 ;
4604-72-2 ;
98-97-5 ;
24005-61-6 ;
103-67-3 ;
5521-61-9 ;
2516-34-9 ;
2719-27-9 ;
123-90-0 ;
6761-50-8 ;
625-43-4 ;
872-64-0 ;
36932-49-7 ;
1528085-68-8 ;
1195533-51-7 ;
13534-79-7
...More


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







-2,2-dimethyl-3,4-dihydro-2H-pyrano[2,3-b]pyridine-7-carboxamide as a PET Imaging Ligand for Metabotropic Glutamate Recept.png)