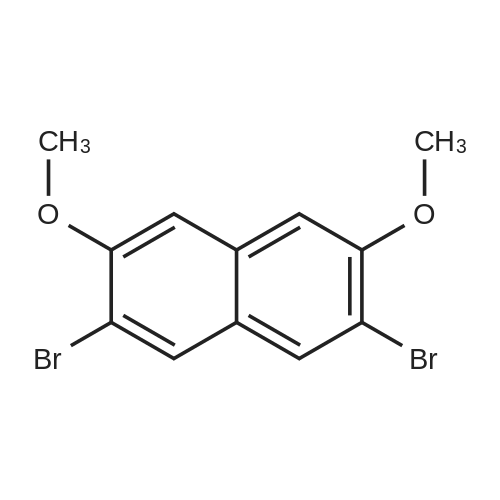
| 83% |
With potassium hydroxide; In dimethyl sulfoxide; at 20℃; for 3h;Cooling with ice; |
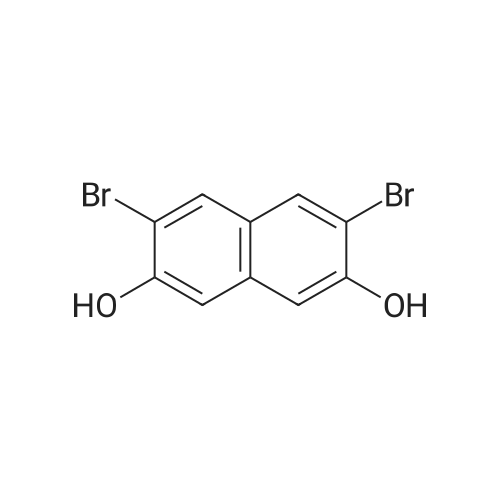
0.47 mol of 3,6-dibromonaphthalene-2,7-diol at room temperature, dissolve 800 ml of DMSO (dimethylsulfoxide) in 150 g. In ice bath KOH 3.77mol, 212g was slowly added and then 1.93mol, methyl iodide (methyl iodide) 275g was added carefully not to increase the internal temperature. After the addition was completed, the mixture was raised to room temperature and further stirred for 3 h. Then, 2.0 L of EtOH (ethanol) was added thereto, and 2.0 L of H 2 O was added sequentially, followed by stirring for 1 hour. Thereafter, the solid obtained by filtration was washed with 1 L of EtOH and dried in an oven. [Intermediate 1-1] 0.39 mol, 135.6 g (yield 83%) were obtained. |
| 83% |
With potassium hydroxide; In dimethyl sulfoxide; at 20℃; for 3h;Cooling with ice; |
0.47 mol of 3,6-dibromonaphthalene-2,7-diol at room temperature,Dissolve 800 ml of DMSO (dimethylsulfoxide) in 150 g.3.77mol and 212g of KOH were slowly added to the ice bath, and 1.93mol and 275g of methyl iodide were added with caution not to increase the internal temperature rapidly.After the addition was completed, the mixture was raised to room temperature and further stirred for 3 h. Then, 2.0 L of EtOH (ethanol) was added thereto, and 2.0 L of H 2 O was sequentially added thereto, followed by stirring for 1 hour.Thereafter, the solid obtained by filtration was washed with 1 L of EtOH, and then dried in an oven to obtain 0.39 mol, 135.6 g (yield 83%) of the title compound [Intermediate 1-1]. |
| 77% |
With potassium carbonate; In acetone; at 20℃; for 4h;Inert atmosphere; |
A solution of 3,6-dibromo-2,7-dihydroxynaphthalene (7, 3.00 g, 9.43 mmol), potassium carbonate (3.91 g, 28.3 mmol) and methyl iodide (1.71 mL, 28.3 mmol) dissolved in anhydrous acetone (100 mL) were stirred at room temperature under argon for 4 h. The reaction mixture was filtered through a Celite pad to remove the inorganics, which was washed with DCM (100 mL). The combined organic filtrate and washings were washed with 10% HCl (2*50 mL), water (2*50 mL), dried (MgSO4) and concentrated in vacuo. The crude solid was recrystallized from ethanol to afford the title compound as fine white needles (2.50 g, 77%), mp 177-178 C (lit. 30 mp 176.5-178 C). 1H NMR (300 MHz, CDCl3) delta 7.88 (s, 2H), 7.05 (s, 2H), 3.98 (s, 6H); 13C NMR (75 MHz, CDCl3) delta 154.3, 133.9, 131.0, 125.3, 111.4, 105.7, 56.2; IR (ATR) (cm-1) 2994, 2940, 2869, 2858, 1618, 1588, 1491, 1464. HRMS (EI+, 70 eV); m/z: [M]+ calcd for C12H10Br2O2 343.9048, found 343.9059. Anal. Calcd for C12H10Br2O2: C, 41.65; H, 2.91; Br, 46.18. Found: C, 41.55; H, 2.85; Br, 46.05. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping