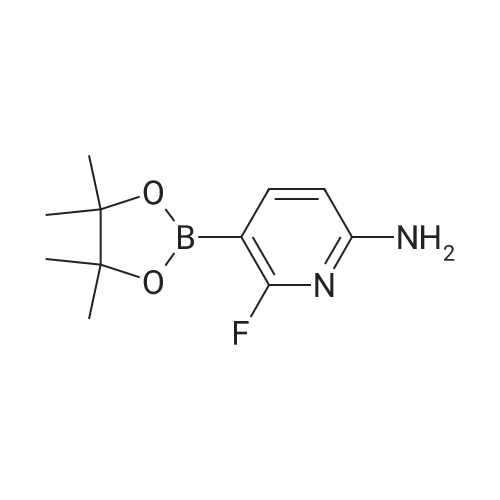
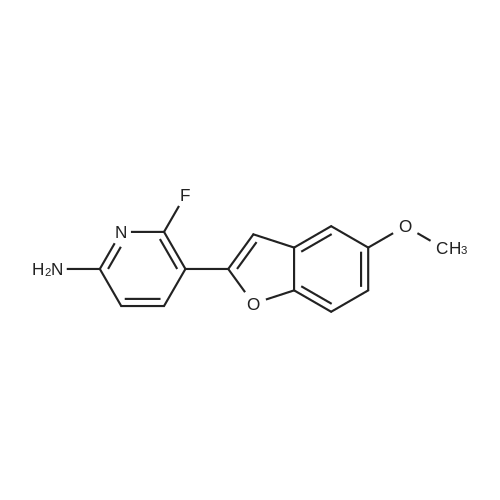
| 64.56% |
With N-Bromosuccinimide; In acetonitrile; at 25℃; for 6.0h; |
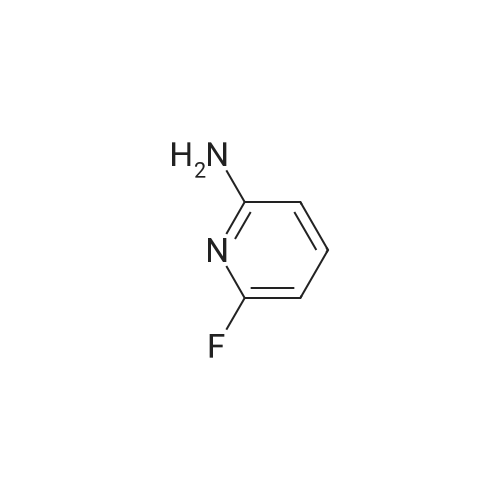
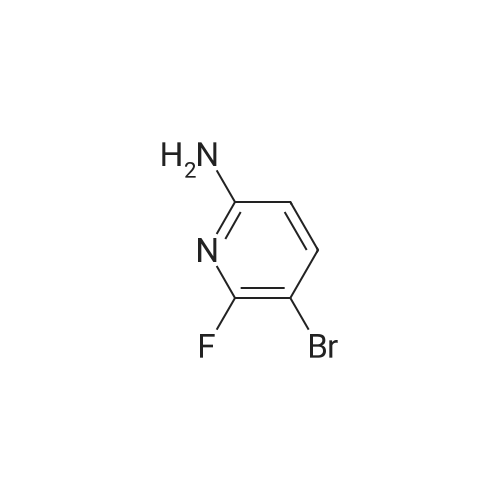
Into a lOOO-mL round-bottom flask, was placed 6-fluoropyridin-2-amine (30 g, 267.601 mmol, 1 equiv), CH ,CN (500 mL), NBS (52 g, 292.161 mmol, 1.09 equiv). The resulting solution was stirred for 6 hr at 25 degrees C. The resulting solution was diluted with 1000 mL of water. The resulting solution was extracted with 3x500 mL of ethyl acetate and the organic layers combined. The resulting mixture was washed with 2 x2000 ml of brine. The mixture was dried over anhydrous sodium sulfate and concentrated under vacuum. The crude product was re-crystallized from PE:EA in the ratio of 5:1. The solids were collected by filtration. This resulted in 33 g (64.56%) of 5- bromo-6-fluoropyridin-2-amine as a white solid. 1H NMR (300 MHz, Chlorofom /, ppm) d 7.61 (t, / = 8.6 Hz, 1H), 6.28 (dd, / = 8.3, 1.4 Hz, 1H), 4.45 (s, 2H). |
| 40% |
With N-Bromosuccinimide; In chloroform; at 20℃; for 16.0h; |
To a 250 mL round bottom flask, were added 6-fluoro-2-aminopyridine (3 g, 0.0267 mol) and chloroform (90 mL). To the same flask, N-bromosuccinimide (5 g, 0.028 mol) was added. The reaction mixture was stirred at room temperature for 16 h. The reaction mixture was diluted with chloroform and washed with water. The organic layer was separated, washed with brine and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to get the title compound [2 g, 40 %]. *H NMR (300 MHz, CDC13): delta 7.62 (t, = 9.0 Hz, 1H), 6.27 (m, 1H), 4.58 (s, 2H); LC-MS: 193.1 [M+2H]+. |
| 22% |
|
[0269] To a solution of 6-fluoro-2-pyridylamine (1.0 g, 8.93 mmol) in chloroform(55 mL) was added N-bromosuccinimide (1.59 g, 8.93 mmol). The solution was stirred in the dark for 15 hours, at which time it was added to CH2Cl2 (200 mL) and IN NaOH (50 mL). Upon mixing, the layers were separated and the organic layer was washed with NaCl(sat.) (50 mL), dried over Na2SO4, filtered and concentrated. The crude material was purified by SiO2 chromatography (25% EtOAc/ hexanes) yielding 5-bromo-6-fluoro-2- pyridylamine (386 mg, 22%). LCMS (m/z): 190.9/192.9 (MH+); 1HNMR (CDCl3): delta 7.59 (t, J= 8.7 Hz, IH), 6.25 (dd, J= 8.1, 1.2 Hz, IH), 4.58 (bs, IH). |
| 22% |
|
Synthesis o -bromo-6-fluoro-2-pyridylamine [00108] To a solution of 6-fluoro-2 -pyridylamine (1.0 g, 8.93 mmol) in chloroform (55 mL) was added N-bromosuccinimide (1.59 g, 8.93 mmol). The solution was stirred in the dark for 15 hours, at which time it was added to CH2CI2 (200 mL) and IN NaOH(50 mL). Upon mixing, the layers were separated and the organic layer was washed with NaCl(Sat.) (50 mL), dried over Na2S04, filtered and concentrated. The crude material was purified by Si02 chromatography (25% EtOAc/ hexanes) yielding 5-bromo-6-fiuoro-2- pyridylamine (386 mg, 22%). LCMS (m/z): 190.9/192.9 (MH ); ? NMR (CDC13): delta 7.59 (t, J = 8.7 Hz, 1H), 6.25 (dd, J= 8.1, 1.2 Hz, 1H), 4.58 (bs, 1H). |
|
With N-Bromosuccinimide; In acetonitrile; for 4.0h; |
To a stirred solution of 6-fluoro-pyridin-2-ylamin (1.0 g, 8.93 mmol) in acetonitrile (50 mL), protected from light and under nitrogen atmosphere, N-bromosuccinimide (0.79 g, 4.46 mmol) was added. After 1 hour, an additional portion of N-bromosuccinimide (0.79 g, 4.46 mmol) was added and the stirring was continued for 3 hours. The volatiles were removed under reduced pressure and the crude material was purified by flash column chromatography using a gradient of 25% to 30% ethyl acetate in hexane to give 5-bromo-6-fluoro-pyridin-2-ylamine (1.45 g) as a white solid. 1H NMR (400 MHz, CHLOROFORM-d) delta: 7.60 (t, J=8.59 Hz, 1H) 6.15-6.36 (m, 1H) 4.58 (br. s., 2H) ESMS: m/z 193.34 [M+1]+ for 81Br isotope |
|
With N-Bromosuccinimide; In acetonitrile; at 10 - 20℃; for 1.5h; |
NBS (50. Og, 280.99mmol) was added slowly to 6-fluoropyridin-2-amine (30g, 267.61mmol) in MeCN (300mL) cooled to 10-20C over a period of 30 minutes. The resulting solution was stirred at ambient temperature for 60 minutes then the solvent removed under reduced pressure. The residue was diluted with water, the precipitate collected by filtration, washed with water (200mL) and dried under vacuum to afford the desired material (50. Og, 98%) as a white solid, which was used without further purification. NMR Spectrum: 1H MR (300MHz, DMSO-d6) delta 6.29 (1H, d), 6.57 (2H, bs), 7.65 (1H, t). Mass Spectrum: m/z (ES+)[M+H]+ = 191. |
|
With N-Bromosuccinimide; In acetonitrile; at 10 - 30℃; for 1.5h;Inert atmosphere; |
NBS (50. Og, 280.99mmol) was added slowly to 6-fluoropyridin-2-amine (30 g, 267.61 mmol) in MeCN (300 mL) cooled to 10-20C over a period of 30 minutes. The resulting solution was stirred at ambient temperature for 60 minutes then the solvent removed under reduced pressure. The residue was diluted with water, the precipitate collected by filtration, washed with water (200 mL) and dried under vacuum to afford the desired material (50.0 g, 98%) as a white solid, which was used without further purification. NMR Spectrum: JH NMR (300MHz, DMSO-d6) delta 6.29 (1H, d), 6.57 (2H, bs), 7.65 (1H, t). Mass Spectrum: m z (ES+)[M+H]+ = 191. |
|
With N-Bromosuccinimide; |
Example 59 Synthesis of 6-fluoropyridin-2-amine. A mixture of 6-fluoropyridin-2-amine (3 g, 26.8 mmol) and N-bromosuccinimide (5.25 g, 29.5 mmol) in dry CH3CN (20 mL) was stirred at 0 C. for 2 h. Water (100 mL) was added and the mixture was extracted with EtOAc (100 mL*3). The combined organic layers were concentrated and purified by silica gel chromatography (EA/PE=1/5) to give 5-bromo-6-fluoropyridin-2-amine (4.31 g, yield: 84.2%) as a white solid. ESI-MS [M+H]+: 191.0. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping