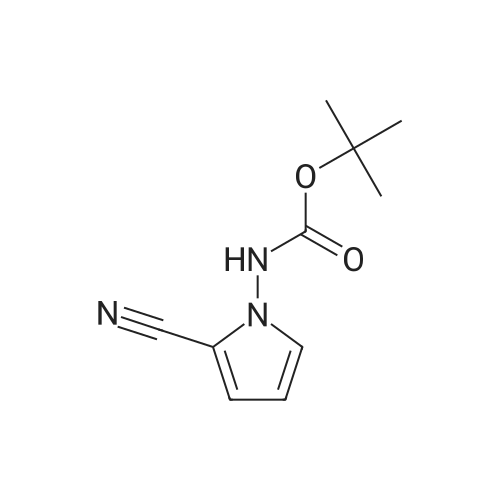
| 77% |
In N,N-dimethyl-formamide; acetonitrile; at 0 - 5℃; for 1.83333h; |
A 2L, 3 -neck RB was fitted w/ stir bar, N2 inlet, rubber septum low-temp, thermometer and ice/acetone cooling bath. Pyrrol- 1-yl-carbamic acid tert-butyl ester (99.0 g, 0.543 mol) was added to the reactor, dissolved w/ anhydrous acetonitrile (700 mL) and the stirred solution was cooled to 0 C. Chlorosulfonyl isocyanate (49.7 mL, 0.57 mol) was added dropwise via syringe (maintaining an internal temp, below 5 0C); after ~ 20 minutes a suspension was observed. After 45 minutes N,N-dimethylformamide (anhydrous, 100 mL) was added dropwise via addition funnel (keeping internal temp, below 5 0C) and the reaction mixture became a solution. Stirring @ 0 0C was continued for 45 minutes, then the reaction was allowed to warm to RT; monitoring by TLC (silica gel, 1:3 ethyl acetate/hexane, UV, ninydrin stain) of a quenched sample indicated that the reaction had progressed to completion. The mixture was poured onto ice (~2L) and stirred with addition of EtOAc (2L). The layers were separated and the organic layer was dried over magnesium sulfate. The dried solution was filtered through a pad of 30/40 Magnesol and the filtrate was concentrated to dryness in vacuo, then the residue was dissolved in a minimum volume of dichloromethane and chromatographed on a plug of silica gel, eluting with ethyl acetate/hexane, 0-50 % ethyl acetate. The clean, product-containing fractions were combined and concentrated to dryness in vacuo, to afford the desired product as a white solid, 69.8 g (62%). A somewhat impure fraction provided additional material, 16.8 g (15%), bringing the total recovery to 86.6 g, (77%). 1H-NMR (CD3OD): £7.01 (dd, IH, J = 3.0, 1.6 Hz), 6.82 (dd, IH, / = 4.4, 1.7 Hz), 6.19 (dd, IH, J=4.2, 2.9 Hz), 4.88 (s, IH,H2O+NH-), 1.50 (br s, 9H, HN-BOC); MS: LC/MS (+esi), m/z=207.9 [M+H] |
| 66% |
|
g. To a stirred solution of tert-butyl lH-pyrrol-1-ylcarbamate 12 (40 g, 219.52 mmol), in acetonitrile (350 mL) was added chlorosulfonyl isocyanate (32.62 g, 230.50 mmol) slowly at 0 C and continued stirring at 0 0C for 30 min. To the solution N, N-dimethyl formamide (40 mL) was added below 5 0C and continued stirring at 0 0C for 1 hr. The reaction mixture was poured into a mixture of crushed ice (1 L) and ethyl acetate (1 L). The layers were separated and the organic layer was washed with water (500 mL), brine (250 mL), dried and concentrated in vacuum to furnish crude (43 g) product. The crude was purified by flash chromatography (silica gel, eluting with ethyl acetate in hexane 0-50%) to afford pure tert-butyl 2-cyano-lH-pyrrol-l-ylcarbamate 13 (30 g, 66 %) as a colorless solid. 1H NMR (300 MHz, DMSO) delta 10.80 (s, IH, D2O exchangeable), 7.23 (dd, J = 1.7, 2.9, IH), 6.94 (dd, J= 1.7, 4.3, IH), 6.20 (dd, J= 2.9, 4.3, IH), 1.45 (s, 9H). HPLC (Zorbax SBC3, 3.0 x 150 mm, 5 mum, with ZGC SBC3, 2.1 x 12.5 mm guard cartridge. Mobile phase: 0.1 M ammonium acetate/ Acetonitrile) Rt = 16.216, (98.14 %). Analysis: CaIc for C10H13N3O2: C, 57.95; H, 6.32; N, 20.27 Found: C, 58.02; H, 6.45; N, 20.18. |
| 66% |
|
To a stirred solution of tert-butyl lH-pyrrol-l-ylcarbamate 12c (40 g, 219.52 mmol), in acetonitrile (350 mL) was added chlorosulfonyl isocyanate (32.62 g, 230.50 mmol) slowly at 0 C and continued stirring at 0 C for 30 min. To the solution N, N-dimethyl formamide (40 mL) was added below 5 C and continued stirring at 0 C for 1 hr. The reaction mixture was poured into a mixture of crushed ice (1 L) and ethyl acetate (1 L). The layers were separated and the organic layer was washed with water (500 mL), brine (250 mL), dried and concentrated in vacuum to furnish crude (43 g) product. The crude was purified by flash chromatography (silica gel, eluting with ethyl acetate in hexane 0-50%) to afford pure tert-butyl 2-cyano-lH-pyrrol-l- ylcarbamate 12d (30 g, 66 %) as a colorless solid. 1H NMR (300 MHz, DMSO) delta 10.80 (s, 1H, D20 exchangeable), 7.23 (dd, J= 1.7, 2.9, 1H), 6.94 (dd, J= 1.7, 4.3, 1H), 6.20 (dd, J= 2.9, 4.3, 1H), 1.45 (s, 9H). HPLC (Zorbax SBC3, 3.0 x 150 mm, 5 mu?iota, with ZGC SBC3, 2.1 x 12.5 mm guard cartridge. Mobile phase: 0.1 M ammonium acetate/ Acetonitrile) Rt = 16.216, (98.14 %). Analysis: Calc for C10H13N3O2: C, 57.95; H, 6.32; N, 20.27 Found: C, 58.02; H, 6.45; N, 20.18. |
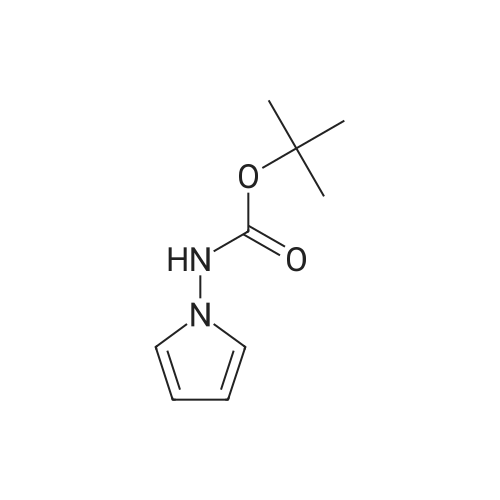
| 66% |
|
Step 1:To a stirred solution of tert-butyl hydrazinecarboxylate 22b (50 g, 412.37 mmol) and 2,5-dimethoxytetrahydrofuran 22a (54.5 g, 412.37 mmol) in dioxane (300 mL) was added aqueous hydrochloric acid (5 mL, 2N). The reaction was set up using a dean-stark apparatus and heated at 90 C for 20 h. Reaction mixture was cooled to 20 C, neutralized with saturated sodium bicarbonate (18 mL) and filtered to remove inorganics. The filtrate was concentrated in vacuum and triturated with ether. The solid obtained was collected by filtration to furnish on drying tert-butyl lH-pyrrol-l-ylcarbamate 22c (43 g, 57.2%) as a yellow brown solid. 1H NMR (300 MHz, CD3OD) ? 6.62 (t, J= 2.3, 2H), 6.02 (t, J= 2.3, 2H), 1.48 (s, 9H); MS (ES+): 181.1 (M _1). Analysis: Calculated for C9Hi4N202: C, 59.32; H, 7.74; N, 15.37. Found: C, 59.32; H, 7.65; N, 15.02. Step 2:To a stirred solution of tert-butyl lH-pyrrol-l-ylcarbamate 22c (40 g, 219.52 mmol), in acetonitrile (350 mL) was added chlorosulfonyl isocyanate (32.62 g, 230.50 mmol) slowly at 0 C and continued stirring at 0 C for 30 min. To the solution N, N-dimethyl formamide (40 mL) was added below 5 C and continued stirring at 0 C for 1 hr. The reaction mixture was poured into a mixture of crushed ice (1 L) and ethyl acetate (1 L). The layers were separated and the organic layer was washed with water (500 mL), brine (250 mL), dried and concentrated in vacuum to furnish crude (43 g) product. The crude was purified by flash chromatography (silica gel, eluting with ethyl acetate in hexane0-50%) to afford pure tert-butyl 2-cyano-lH-pyrrol-l-ylcarbamate 22d (30 g, 66 %>) as a colorless solid. 1H NMR (300 MHz, DMSO-d6) ? 10.80 (s, 1H, D20 exchangeable), 7.23 (dd, J= 1.7, 2.9, 1H), 6.94 (dd, J= 1.7, 4.3, 1H), 6.20 (dd, J= 2.9, 4.3, 1H), 1.45 (s, 9H). Analysis: Calculated for Ci0Hi3N3O2: C, 57.95; H, 6.32; N, 20.27. Found: C, 58.02; H, 6.45; N, 20.18. Step 3:To a stirred solution of tert-butyl 2-cyano-lH-pyrrol-l-ylcarbamate 22d (5 g, 24.12 mmol) in ethyl alcohol (100 mL) was added concentrated aqueous ammonium hydroxide solution (50 mL) at 20 C followed by hydrogen peroxide (7.4 mL, 72.38 mmol, 30 % in water) slowly at 20 C and stirred at the same temperature for 16 h. Reaction mixture was concentrated in vacuum and diluted with ethyl acetate (150 mL), washed with water (2 x 50 mL). The aqueous layer was extracted with ethyl acetate (150 mL). The combined ethyl acetate layers were washed with water (100 mL), brine (50 mL), dried, filtered, and concentrated in vacuum. The residue obtained was crystallized from diisopropyl ether and hexane to afford tert-butyl 2-carbamoyl-lH-pyrrol-l-ylcarbamate 22e (4.0 g, 73.6%) as a colorless solid. 1H NMR (300 MHz, DMSO-d6) ? 9.89 (s, 1H, D20 exchangeable), 7.31 (d, J = 38.5, 1H), 6.84 (dd, J= 1.9, 2.8, 2H, lH is D20 exchangeable), 6.76 (dd, J = 1.9, 4.2, 1H), 5.97 (dd, J= 2.8, 4.2, 1H), 1.40 (s, 9H); Analysis: Calculated for Ci0Hi5N3O3: C, 53.32; H, 6.71 ; N, 18.65. Found: C, 53.40; H, 6.74; N, 18.55. Step 4:To a solution of tert-butyl 2-carbamoyl-lH-pyrrol-l-ylcarbamate 22e (2g, 8.87 mmol) in dichloromethane (15 mL) was added trifluoro acetic acid (15 mL) at 20 C and stirred for 30 min. The reaction mixture was concentrated to dryness to remove excess trifluoroacetic acid and diluted with dichloromethane. Triethylorthoformate (30 mL) was added to the residue and was heated to 79 C overnight. Reaction mixture was concentrated to dryness and triturated with hexanes, the solid obtained was collected by filtration dried in vacuum to give crude pyrrolo[l ,2-f][l ,2,4]triazin-4-ol 22f (1.1 g, 91%) as a dark brown solid. 1H NMR (300 MHz, DMSO-d6) ? 11.63 (s, 1H, D20 exchangeable), 7.83 (d, J= 4.0, 1H), 7.59 (dd, J= 1.7, 2.6, 1H), 6.89 (dd, J= 1.6, 4.3, 1H), 6.54 (dd, J = 2.7, 4.3, 1H); MS (ES+): 136.2 (M + l). Step 5:The stirred solution of pyrrolo[l ,2-f][l ,2,4]triazin-4-ol 22f (1 g, 7.40 mmol), benzyltriethylammonium chloride (3.29 g, 14.80 mmol), and N,N-dimethylaniline (1.35 g, 1 1.10 mmol) in acetonitrile (25 mL) was heated to 80 C and at this temperature phosphorous oxy chloride (6.88 g, 44.40 mmol) was added and stirred at 80 C for 16 h. The reaction was concentrated to remove acetonitrile and phosphorus oxy chloride. The reaction was quenched by adding ice water (20 mL) and extracted with ethyl acetate (2 x 100 mL). The ethyl acetate extracts were combined washed with hydrochloric acid (1 N, 30 mL), water (50 mL), saturated sodium bicarbonate (1 x 20 mL), water (50 mL), brine (20 mL) dried and concentrated. The crude residue was purified by flash chromatography (silica gel, eluting with ethyl acetate in hexanes (0 to 5 %)) to furnish pure4-chloropyrrolo[l,2-fJ[l,2,4]triazine 22g (0.7 g, 61.6 %) as a colorless oil, which solidified on standing in refrigerator. 1H NMR (300 MHz, DMSO-d6) ? 8.44 (s, 1H), 8.27 (dd, J = 1.5, 2.5, 1H), 7.12 (qd, J= 2.0, 4.6, 2H). Step 6:A solution of 2-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)aniline (10m) (2.3 g, 9.8 ... |
|
|
Example 2: tert-butyl (2-cyano- lH-pyrrol- 1 -yl)carbamateUnder an atmosphere of nitrogen, the suspension of the compound (322g) prepared in Example 1 in acetonitrile (2L) was added into a flask. The solution was cooled to -70C. Chlorosulfonyl isocyanate (162mL, 1.86mol) was dropped into the solution and was stirred for an hour at O0C. Then, N,N-dimethylformamide (DMF) (325mL) was dropped into the mixture and stirred for an hour at 5C. The reaction solution was poured into iced water (4L) and the aqueous layer was extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate and solvent removed under reduced pressure. The residue was purified by column chromatography on silica gel (hexane: ethyl acetate = 1:0 - >;4:1 ->; 3:1 - >; 3:2). The obtained solids were washed in hexane/diisopropylether (1:1; 40OmL) to obtain the title compound (148g) as a white solid. The filtrate was concentrated and washed in hexane/diisopropylether (10:1; 30OmL) to obtain the title compound (112g) as a white solid. A total of 26Og of the title compound having the following physical data was obtained.TLC: Rf 0.42 (hexane : ethyl acetate = 3:1);1HNMR (300 MHz, CDCl3) delta ppm 1.52 (s, 9 H) 6.19 (dd, J=4.4, 2.9 Hz, 1 H) 6.79 (dd, J=4.4, 1.8 Hz, 1 H) 6.90 (dd, J=2.9, 1.8 Hz, 1 H) 7.26 (s, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping