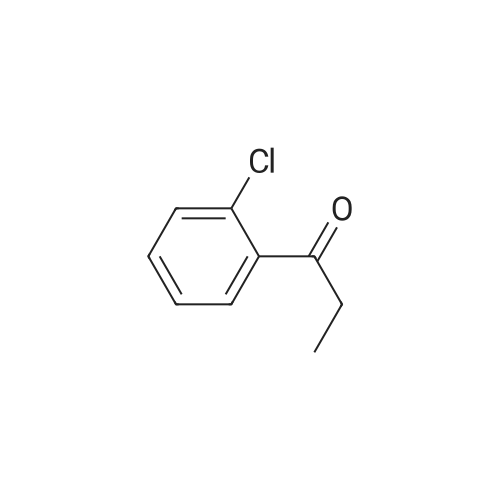
| 96% |
With formic acid; C30H31ClN2O2RuS; triethylamine; at 60℃; for 1.5h;Inert atmosphere; |
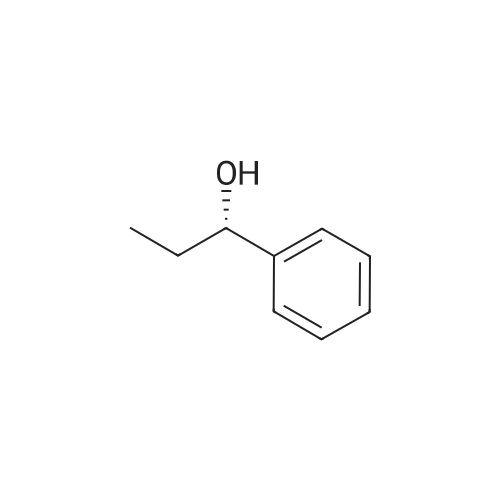
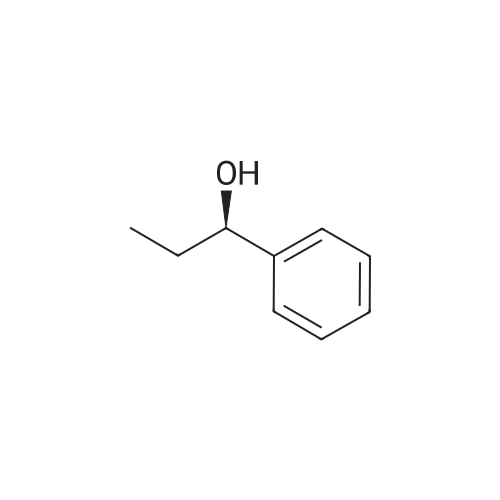
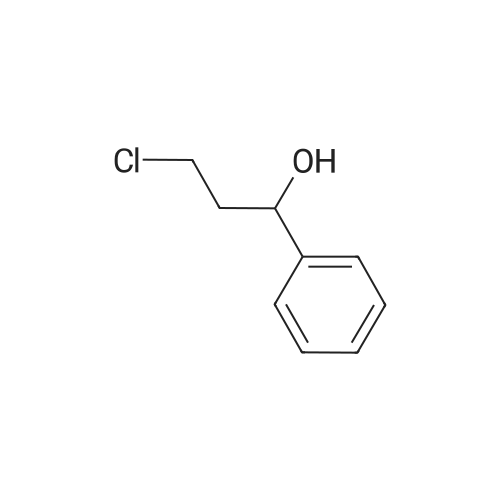
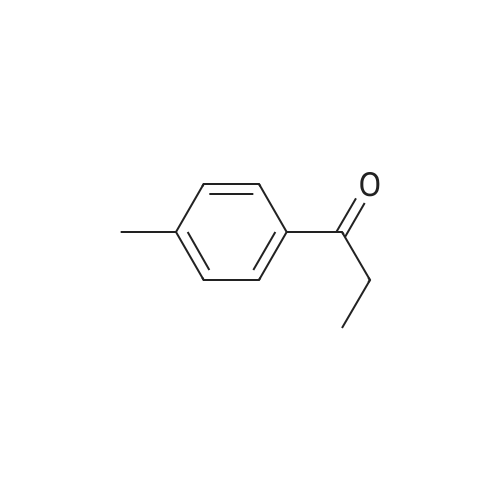
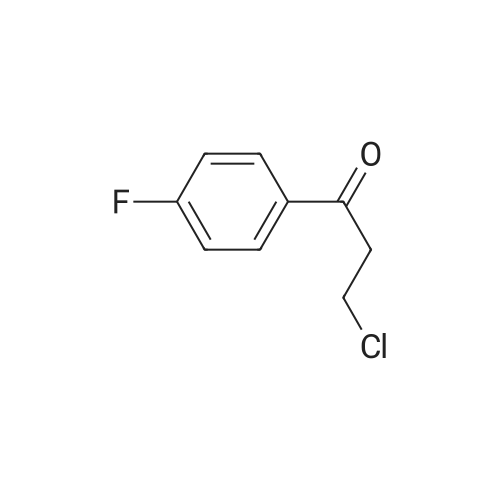
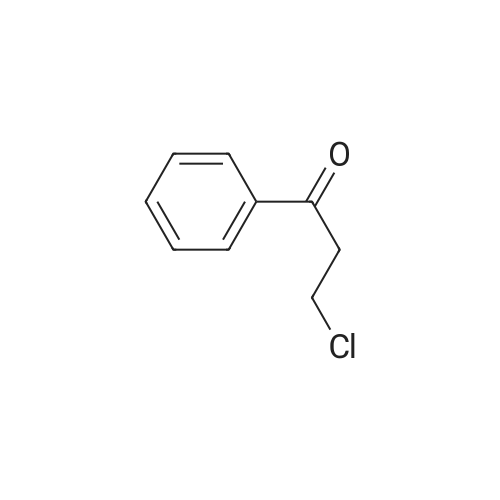
This compound is known [17]. From 3-chloropropiophenone 13: A degassed solution of 3-chloropropiophenone (170 mg, 1.01 mmol, 1.0 eq) and (S,S)-2 (3.1 mg, 0.005 mmol, 0.5%) in FA/TEA (5:2, 0.5 mL) was stirred at 60 C for 1.5 h. The mixture was diluted with ethyl acetate (5 mL) and quenched with NaHCO3 (sat., 5 mL), the aqueous layer was extracted further with ethyl acetate (2 5 mL) and the organic extracts dried over Na2SO4 and concentrated to give a brown oil. The crude was dissolved in diethyl ether and passed through a silica plug to yield the product 14 as a red oil (123 mg, 0.97 mmol, 96%) in 100% conv and 95% ee as measured by GC. [α]D22 -43.5 (c 0.35 in CHCl3); lit [17] [α]D22 -43.6 (S) (c 1.0 in CHCl3); δH (300 MHz, CDCl3) 7.29-7.15 (5H, m, Ph), 4.49 (1H, dt, J = 3.2, 6.6 Hz, CHOH), 1.85 (1H, brs, OH), 1.78-1.57 (2H, m, CH2), 0.82 (3H, t, J = 7.4 Hz, CH3); δC (75 MHz, CDCl3) 143.9, 127.8, 126.9, 125.4, 75.4, 31.3, 9.5; Chiral GC; (CP-Chirasil-Dex-Cβ, 25 m 0.25 mm x 0.25 μm column, oven: hold 12 min at 125 C, then ramp 1 C/min, final temp 145 C, inj.: split 220 C, det.: FID 250 C, 18 Psi He), retention times: 11.2 (R) and 11.4 (S) minutes. From phenyl vinyl ketone 15: Compound 14 could also be prepared with 100% conversion and 95% ee with the same method, starting from 15 (prepared by elimination of HCl from 3-chloropropriophenone [18], 126 mg, 0.95 mmol, 1 eq), (S,S)-2 catalyst (3.5 mg, 0.006 mmol, 0.5%) and FA/TEA (5:2, 0.5 mL). The product was isolated as a clear oil (103 mg, 0.76 mmol, 79%). Attempted reduction of 1-phenylprop-2-en-1-ol 17. Application of the same method to commercially available α-vinylbenzyl alcohol 17 (134 mg) and (S,S)-2 catalyst (3.3 mg, 5 μmol, 0.5%) in FA/TEA (5:2. 0.5 mL) gave no reaction in 1.5 h at 60 C. |
| 100%Chromat. |
With yeast culture of Rhodotorula marina KCh 77; In acetone; at 25℃; for 144h;Microbiological reaction; |
General procedure: Erlenmeyer flasks (300 ml), each containing 100 ml of the mediumconsisting of 3 g glucose and 1 g aminobac dissolved in water,were inoculated with a suspension of microorganisms and then incubated for 3-7 days at 25 C on a rotary shaker (190 rpm). After full growth of the culture 20 mg of a substrate dissolved in 1 ml of acetone was added. After 1, 3, 6, 9, 12 h and 1, 3, 6, 9 days of incubation under the above conditions, portions of 5 ml of the transformation mixture were taken out and extracted with CHCl3(3*10 ml). The extracts were dried over MgSO4, concentrated in vacuo, and analyzed by GC. All the experiments were repeatedthree times. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


 alicyclic diamines with antimicrobial activity against multidrug-resistant Gram-positive bacteria.jpg)