Alternatived Products of [ 932-87-6 ]
Product Details of [ 932-87-6 ]
| CAS No. : | 932-87-6 |
MDL No. : | MFCD00015715 |
| Formula : |
C8H5Br
|
Boiling Point : |
- |
| Linear Structure Formula : | BrCC(C6H5) |
InChI Key : | BPVHWNVBBDHIQU-UHFFFAOYSA-N |
| M.W : |
181.03
|
Pubchem ID : | 136737 |
| Synonyms : |
|
Application In Synthesis of [ 932-87-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Upstream synthesis route of [ 932-87-6 ]
- Downstream synthetic route of [ 932-87-6 ]
- 1
-
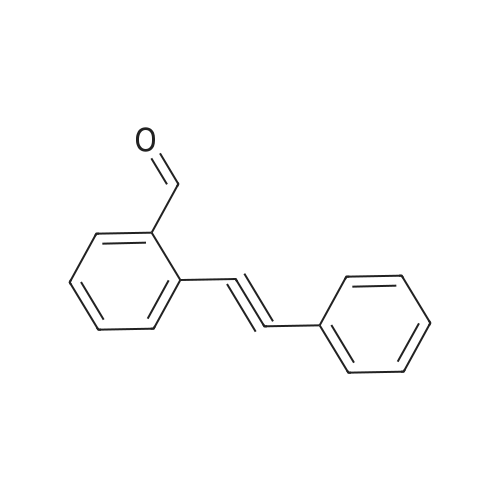 [ 59046-72-9 ]
[ 59046-72-9 ]

-
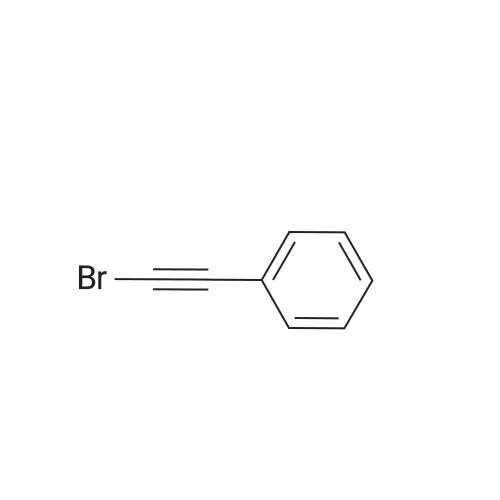 [ 932-87-6 ]
[ 932-87-6 ]

-
2-bromo-3-phenylnaphthalene
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 86% |
With Difluoroacetic acid; copper(II) bis(trifluoromethanesulfonate); In 1,2-dichloro-ethane; at 100℃; for 0.25h;Inert atmosphere; |
Referring to Journal of the American Chemical Society, 2003, 125(36), 10921, a mixture of <strong>[59046-72-9]o-(phenylethynyl)benzaldehyde</strong> (0.5 mmol, CAS No. 59046-72-9) and Cu(OTf)2(5 mol %) in 1,2-dichloroethane (2 ml) were added with (bromoethynyl)benzene (0.6 mmol, CAS No. 932-87-6) and CF2HCO2H (0.5 mmol) successively at room temperature under N2 atmosphere. The resulting mixture was stirred at 100 C. for 15 min and then cooled to room temperature. A saturated aqueous solution of NaHCO3 was added, and the mixture was extracted with ether three times. The combined extracts were washed with brine, dried over MgSO4, and evaporated to leave the crude product, which was purified by silica gel column chromatography using hexane as eluent to give 2-bromo-3-phenylnaphthalene (0.43 mmol) in 86% yield. |
| 86% |
With Difluoroacetic acid; copper(II) bis(trifluoromethanesulfonate); In 1,2-dichloro-ethane; at 100℃; for 0.25h;Inert atmosphere; |
Referring Journal of the American Chemical Society, 2003, 125(36), 10921, To a mixture of <strong>[59046-72-9]o-(phenylethynyl)benzaldehyde</strong> (0.5 mmol, CAS No. 59046-72-9) and Cu(OTf)2 (5 mol%) in 1,2-dichloroethane (2 ml), (bromoethynyl)benzene (0.6 mmol, CAS No. 932-87-6) and CF2HCO2H (0.5 mmol) were successively added at room temperature under an atmosphere of N2. Stirring the resulting mixture at 100 15 minutes and then cooled to room temperature. A saturated aqueous NaHCO3 solution was added and the mixture was extracted three times with ether. Combined extracts were rinsed with Breen, followed by drying and evaporation over MgSO4, by using hexane as an eluent was purified by silica gel column chromatography to give 2-bromo-3-phenyl-naphthalene (0.43 mmol) to yield 86%. |
- 2
-
 [ 932-87-6 ]
[ 932-87-6 ]

-
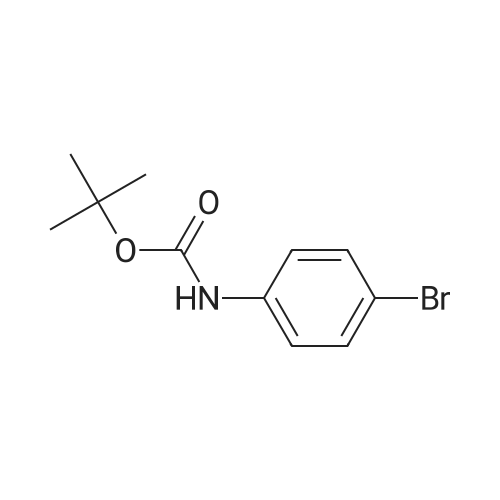 [ 131818-17-2 ]
[ 131818-17-2 ]

-
 [ 1011269-16-1 ]
[ 1011269-16-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With potassium phosphate; 1,10-Phenanthroline; copper(ll) sulfate pentahydrate; In toluene; at 85℃; for 18h;Sealed tube; |
General procedure: To a mixture of tert-butyloxycarbamates (8.0 mmol), K3PO4 (16 mmol), CuSO4*5H2O (0.8 mmol), and 1,10-phenanthroline (1.6 mmol) in a reaction vial was added a solution of bromoalkyne (8.8 mmol) in toluene (15 mL).The reaction mixture was capped and heatedin an oil bath at 85 C for 18 h while being monitored with TLC analysis. Upon completion, the reaction mixture was cooled to room temperature and diluted with EtOAc and filtered through Celite, and the filtrate was concentrated in vacuum. The crude products were purified by silica gel flash chromatography on a silica gel column with petroleum ether (PE) and ethylacetate (EA) as eluent to afford directing products. |
- 3
-
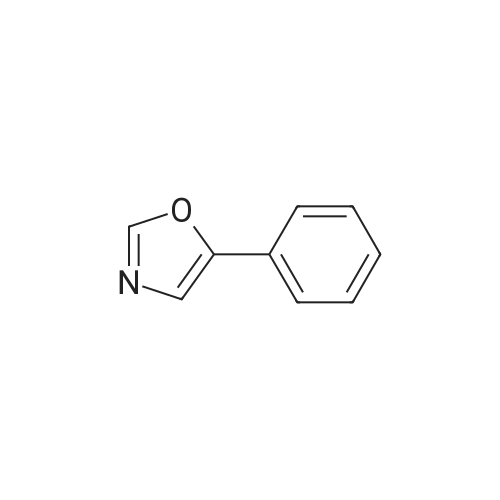 [ 1006-68-4 ]
[ 1006-68-4 ]

-
 [ 932-87-6 ]
[ 932-87-6 ]

-
 [ 1187772-41-3 ]
[ 1187772-41-3 ]
- 4
-
 [ 1006-68-4 ]
[ 1006-68-4 ]

-
 [ 932-87-6 ]
[ 932-87-6 ]

-
 [ 1187772-41-3 ]
[ 1187772-41-3 ]

-
 [ 17064-30-1 ]
[ 17064-30-1 ]
- 5
-
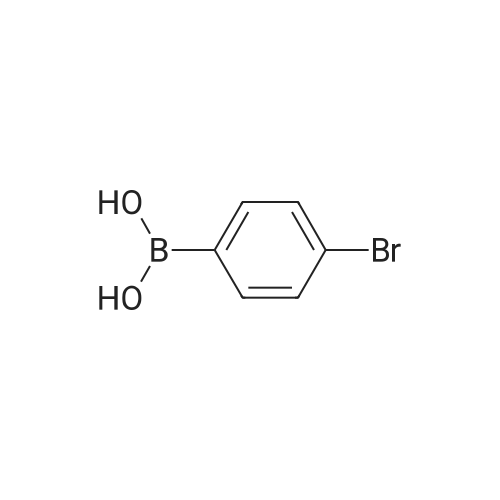 [ 5467-74-3 ]
[ 5467-74-3 ]

-
 [ 932-87-6 ]
[ 932-87-6 ]

-
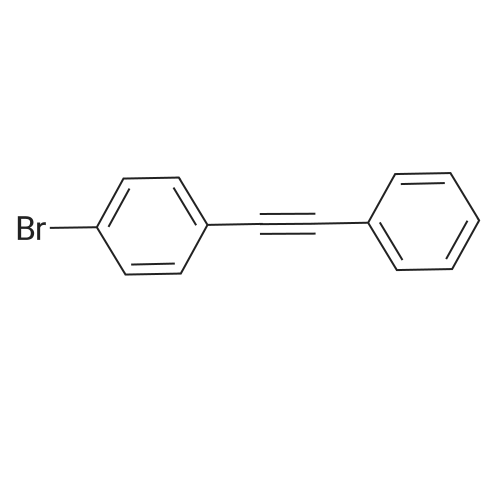 [ 13667-12-4 ]
[ 13667-12-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 93% |
With copper(l) iodide; 8-quinolinol; sodium phosphate; In ethanol; at 80℃; for 24h; |
General procedure: A 5.0 mL of reaction tube was charged with organoboron compound (1.0 mmol), alkynyl bromide (1.0 mmol), CuI (0.10 mmol), 8-hydroxyquinoline (0.20 mmol), ethanol (2.0 mmol). The mixture was stirred at 80 C for 24 h, and then washed with ethyl acetate (3.0 mL×3), the combined ethyl acetate was concentrated under reduced pressure. The obtained residue was purified by flash column chromatography on silica gel (petroleum ether as eluting agent) to give the corresponding pure cross-coupling product. |
- 6
-
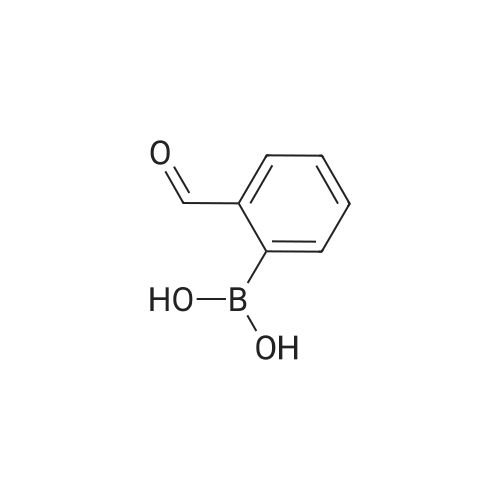 [ 40138-16-7 ]
[ 40138-16-7 ]

-
 [ 932-87-6 ]
[ 932-87-6 ]

-
 [ 59046-72-9 ]
[ 59046-72-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 86% |
With copper(l) iodide; 8-quinolinol; sodium phosphate; In ethanol; at 80℃; for 24h; |
General procedure: A 5.0 mL of reaction tube was charged with organoboron compound (1.0 mmol), alkynyl bromide (1.0 mmol), CuI (0.10 mmol), 8-hydroxyquinoline (0.20 mmol), ethanol (2.0 mmol). The mixture was stirred at 80 C for 24 h, and then washed with ethyl acetate (3.0 mL×3), the combined ethyl acetate was concentrated under reduced pressure. The obtained residue was purified by flash column chromatography on silica gel (petroleum ether as eluting agent) to give the corresponding pure cross-coupling product. |
- 7
-
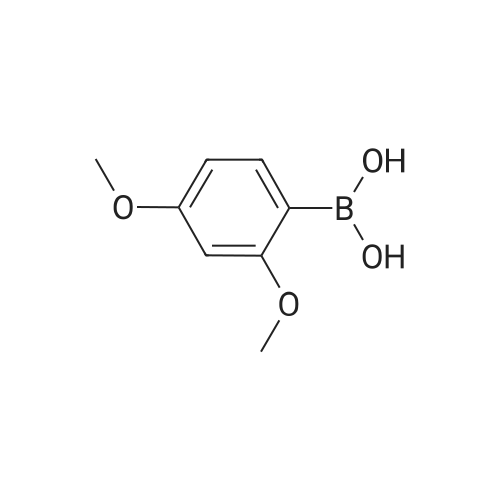 [ 133730-34-4 ]
[ 133730-34-4 ]

-
 [ 932-87-6 ]
[ 932-87-6 ]

-
 [ 78594-14-6 ]
[ 78594-14-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 99% |
With copper(l) iodide; 8-quinolinol; sodium phosphate; In ethanol; at 80℃; for 24h; |
General procedure: A 5.0 mL of reaction tube was charged with organoboron compound (1.0 mmol), alkynyl bromide (1.0 mmol), CuI (0.10 mmol), 8-hydroxyquinoline (0.20 mmol), ethanol (2.0 mmol). The mixture was stirred at 80 C for 24 h, and then washed with ethyl acetate (3.0 mL×3), the combined ethyl acetate was concentrated under reduced pressure. The obtained residue was purified by flash column chromatography on silica gel (petroleum ether as eluting agent) to give the corresponding pure cross-coupling product. |
- 8
-
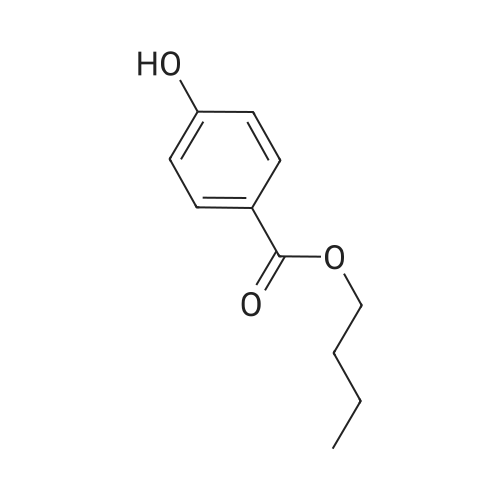 [ 94-26-8 ]
[ 94-26-8 ]

-
 [ 932-87-6 ]
[ 932-87-6 ]

-
C19H19BrO3
[ No CAS ]
- 9
-
 [ 94-26-8 ]
[ 94-26-8 ]

-
 [ 932-87-6 ]
[ 932-87-6 ]

-
 [ 1341216-51-0 ]
[ 1341216-51-0 ]
- 10
-
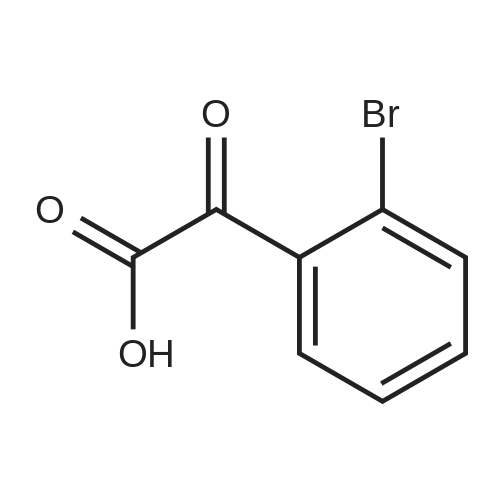 [ 26767-16-8 ]
[ 26767-16-8 ]

-
 [ 932-87-6 ]
[ 932-87-6 ]

-
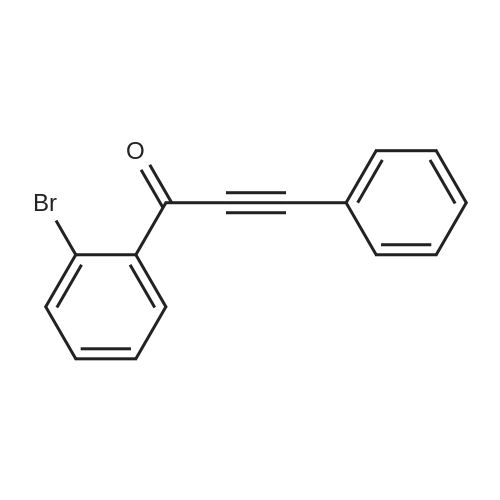 [ 134419-77-5 ]
[ 134419-77-5 ]
- 11
-
 [ 932-87-6 ]
[ 932-87-6 ]

-
 [ 131818-17-2 ]
[ 131818-17-2 ]

-
 [ 7495-11-6 ]
[ 7495-11-6 ]
- 12
-
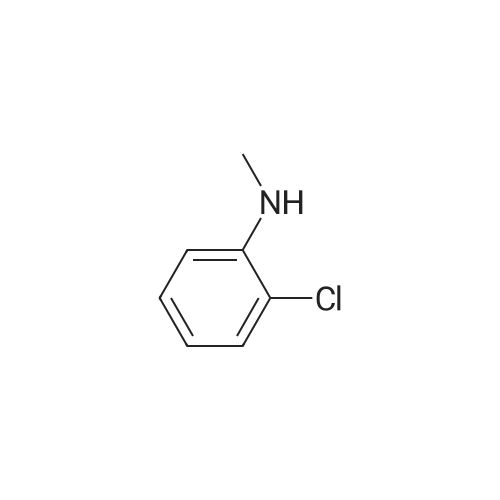 [ 932-32-1 ]
[ 932-32-1 ]

-
 [ 932-87-6 ]
[ 932-87-6 ]

-
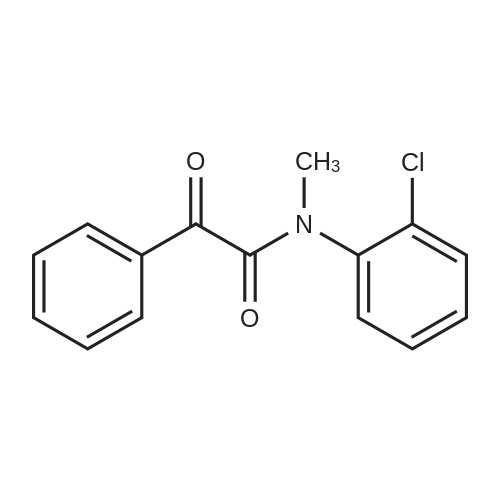 [ 1206913-17-8 ]
[ 1206913-17-8 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping