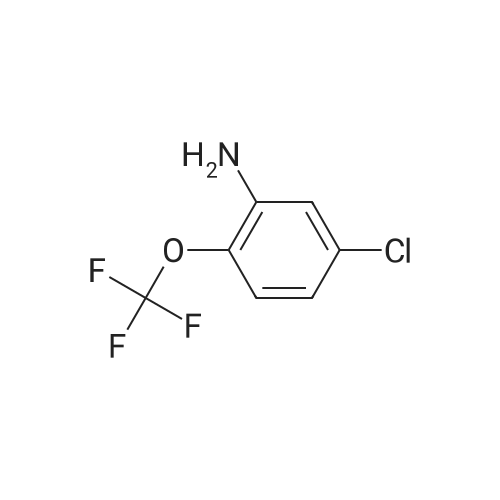
| 91% |
With aluminum (III) chloride; In 1,2-dichloro-ethane; at 0 - 20℃; for 24h; |
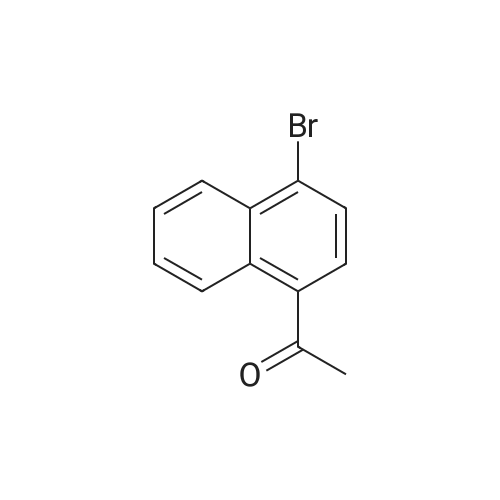
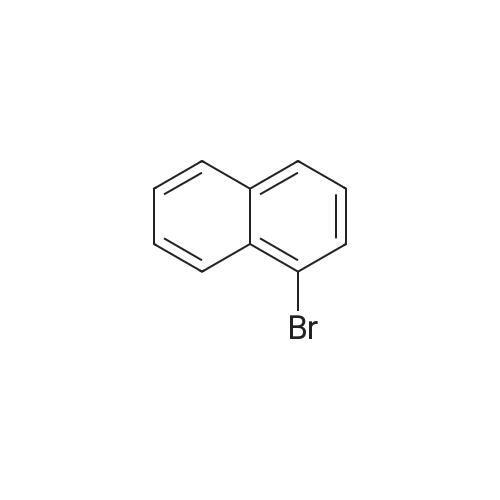
A solution of 1-Bromo-naphthalene (10 g, 48.3 mmol) and acetyl chloride (4.2 ml, 58 mmol) in 1,2-dichloroethane (100 ml) was cooled to 0C and aluminum chloride (14.4 g, 108 mmol) was added portion wise. The mixture was stirred at RT for 24 hours. The reaction mixture was poured into ice-water (100 ml). The two layers were separated and the water layer was extracted with diethyl ether (3 x 150 ml). The combined organic layers were dried over magnesium sulfate, filtered and the solvent was removed under reduced pressure to give an orange colored oil. The 1- (4-bromo-naphtalen-1-yl)-ethanone was purified by flash chromatography (cyclohexane/ethylacetate: 95/5), yielding an yellow oil (91% yield). The 1- (4-bromo-naphtalen-1-yl)-ethanone oxime was prepared according to the procedure described for Compound 22, yielding a white powder (98% yield). Activated zinc dust (24.7 g, 379 mmol) was added portion wise to a suspension of the oxime (10.0 g, 37.9 mmol) in acetic acid (40 ml). The mixture was stirred at RT for 2 hours. The zinc dust was removed by filtration and acetic acid was removed under reduced pressure. Water (100 ml) was added and the pH was adjusted to pH = 13 with 1N NaOH. The water layer was extracted with EtOAc (3 x 100 ml). The combined organic layers were dried over MgS04, filtered and the solvent was removed under reduced pressure, yielding a yellow oil (70% yield). Boc20 (7.1 g, 31.8 mmol) was added to a solution of the amine (6.6 g, 26.5 mmol) in 1,4-dioxane (50 ml). The reaction mixture was stirred at RT for 2 hours. The solvent was removed under reduced pressure and the product was purified by flash chromatography (cyclohexane/EtOAc: 95/5), yielding a yellow powder (75% yield). The bromide (350 mg, 1 mmol) was dissolved in THF (13 ml) /water (2 ml). Potassium acetate (100 mg, 1 mmol), 1,3-bis-diphenylphosphinopropane (9.0 mg, 0.02 mmol) and palladium- (11)-acetate (9.0 mg, 0.04 mmol) were added. The mixture was stirred at 50 atm CO pressure and 150C for 3 hours. The reaction mixture was filtered, the filtrate dried over MgS04 and the solvent was removed under reduced pressure to give a yellow- greenish oil (300 mg). The 4- (l-tert-butoxycarbonylamino-ethyl)-naphthalene-l- carboxylic acid was purified by flash chromatography (DCM/MeOH : 90/10), yielding a white powder (14% yield). The title product was prepared according to the procedure of Compound 31, starting from 4- (1-tert-butoxycarbonylamino-ethyl)-naphthalene-1-carboxylic acid (44 mg) and 4-amino-pyridine (67% yield).'H NMR (300 MHz, , DMSO-d6): 1.64 ppm (d, 3H, J = 6.6 Hz); 5.3 ppm (q, 1H, J = 6.5 Hz), 7.71 ppm (m, 1H), 8.00 ppm (d, 1H, J = 7.7 Hz), 8.32 ppm (m, 1H), 8. 35 ppm (d, 1H, J = 7.3 Hz), 8.81 ppm (d, 2H, J = 7.2 Hz), 12.2 ppm (s, 1H). |
| 91% |
aluminum (III) chloride; In 1,2-dichloro-ethane; at 0 - 20℃; for 24h; |
Section IExample I-IPreparation of Compound 301 and 302General Procedure I-AA solution of 1-Bromo-naphthalene (I-Ia; 2 g, 9.6 mmol) and acetyl chloride (0.84 mL, 11.6 mmol) in 1,2-dichloroethane (30 mL) was cooled to 0 C. and aluminum chloride (2.88 g, 21.6 mmol) was added portion wise. The mixture was stirred at r.t. for 24 hours. The reaction mixture was poured into ice-water (100 mL). The two layers were separated and the aqueous layer was extracted with EtOAc (150 mL×3). The combined organic layers were dried over magnesium sulfate, filtered and the solvent was removed under reduced pressure to give compound I-Ib as an orange oil (2.16 g, yield 91%). 1H NMR (400 MHz, CDCl3) delta 8.6 (m, 1H), 8.3 (m, 1H), 7.8 (d, J=8.0 Hz, 1H), 7.66 (d, J=7.6 Hz, 1H), 7.58 (m, 2H), 2.63 (s, 3H). MS (ESI) m/z (M+H)+ 250. |
| 62% |
With aluminum (III) chloride; In carbon disulfide; at 0 - 20℃; for 120h;Inert atmosphere; |
To a stirred solution of 1.00 g (4.8 mmol) of 1-bromonaphthalene in 15 mL of carbon disulfide at 0 C in a flame-dried flask under N2 was added 0.42 g (5.3 mmol) of acetyl chloride. This solution was stirred at 0 C for 10 min and 0.84 g (6.3 mmol) of AlCl3 was added. The reaction was stirred at 0 C for 3 days followed by 2 days of stirring at ambient temperature. The reaction mixture was poured over ice and concentrated HCl, extracted with ether, washed with NaHCO3 and brine. After drying (MgSO4) the solution was concentrated in vacuo and purified by chromatography (petroleum ether/ether, 95:5) to give 0.75 g (62%) of 1-acetyl-4-bromonaphthalene as a brown oil: 1H NMR (300 MHz, CDCl3) delta 2.74 (s, 3H), 7.65-7.69 (m, 2H), 7.74 (d, J = 6 Hz, 1H), 7.83 (d, J = 6 Hz, 1H), 8.32-8.35 (m, 1H), 8.72-8.75 (m, 1H); 13C NMR (75.5 MHz, CDCl3) delta 30.1, 126.4, 127.5, 127.8, 128.2, 128.4, 128.7, 128.7, 131.2, 132.3, 135.2, 201.0; GC/MS (EI) m/z (rel intensity) 248 (46), 233 (100), 205 (44). The data agree in all respects with those reported previously. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping