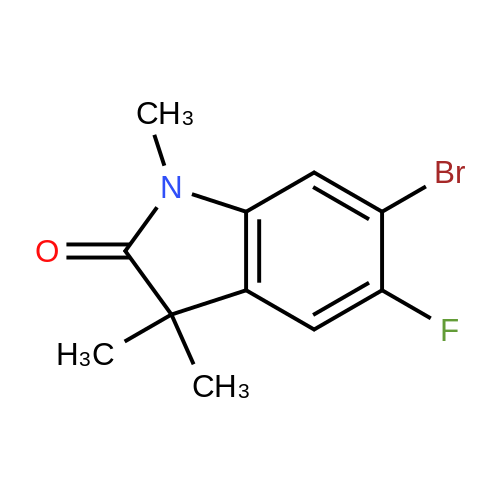
|
|
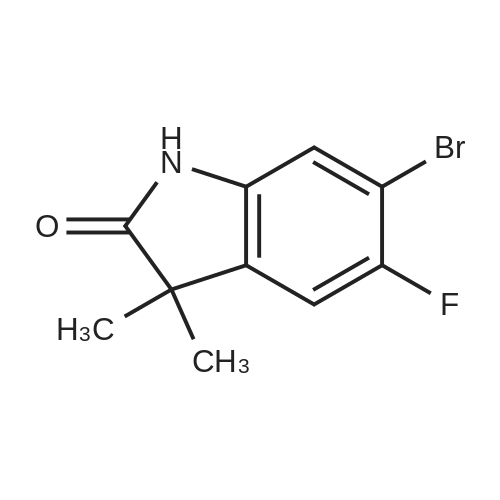
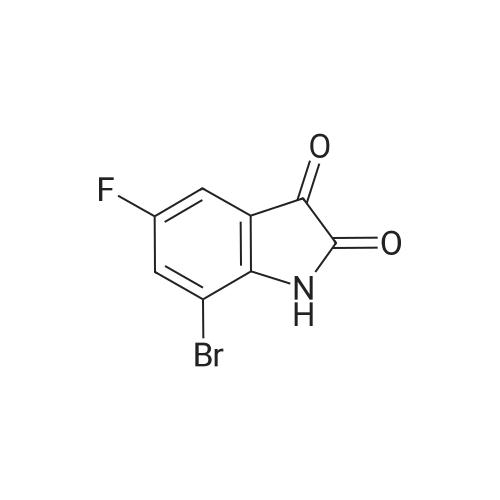
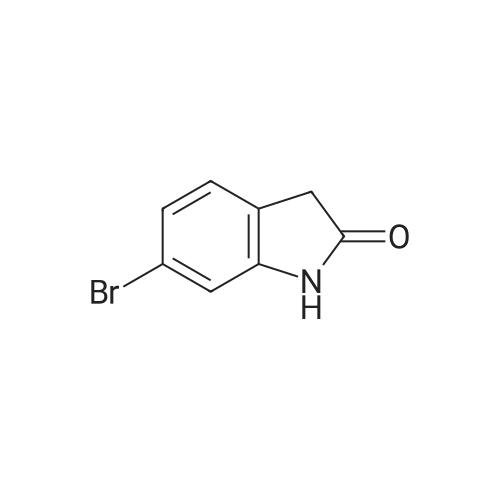
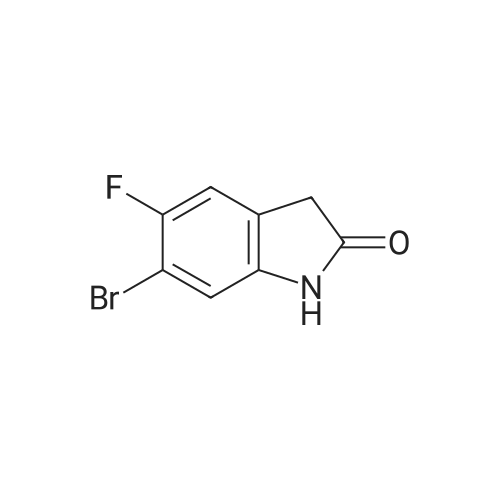
c) 6-Bromo-5-fluoroindolin-2-one A suspension of (4-bromo-5-fluoro-2-nitrophenyl)-acetic acid/ (2-bromo-5-fluoro-4-nitro- phenyl)-acetic acid (2.6: 1 mixture, 37.3 g, 134 mmol) and iron (30.0 g, 537 mmol) in acetic acid (671 ml) was heated to 100C for 7 hours and then cooled to room temperature. Remaining elemental iron was removed with a magnetic rod. Ice water (900 ml) was added to the reaction mixture. The precipitate was filtered off, washed four times with water and then suspended in an ice-cold aqueous solution of 25% HC1 (300 ml) and cone. HC1 (50 ml). After stirring for 10 minutes the precipitate was filtered off and washed four times with water. The precipitate was suspended in a mixture of 1 M aqueous Na2C03 (400 ml) solution and 0.1 M NaOH (100 ml) and stirred for 40 minutes. The precipitate was filtered off and washed four times with 0.1 M aqueous NaOH, three times with water and once with diisopropylether to give title compound as light grey solid (20.5 g). MS ESI (m/z): 228.0/ 230.0 [(M-H)"]. 1H NMR (DMSO-D6, 400 MHz): (ppm) = 10.47 (bs, 1H), 7.31-7.28 (m, 1H), 7.01-6.99 (m, 1H), 3.49 (s, 2H). |
| 20.5 g |
|
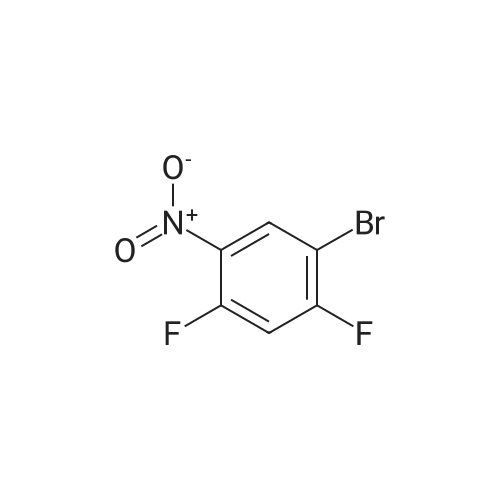
a) 2-(4-Bromo-5-fluoro-2-nitro-phenyl)-malonic acid dimethyl ester/ 2-(2-Bromo-5-fluoro-4-nitro-phenyl)-malonic acid dimethyl ester A suspension of NaH (60 % in mineral oil, 20.2 g, 504 mmol) in dioxane (233 ml) was cooled to11 C. A solution of 1-bromo-2,4-difluoro-5-nitrobenzene (50 g, 26.5 ml, 210 mmol) anddimethyl malonate (33.3 g, 28.9 ml, 242 mmol) in dioxane (467 ml) was carefully added at 11 - 14 C within 45 minutes (gas evolution). After completion of the addition the reaction mixture was kept at 12 C for another hour and then warmed to room temperature. After 16 hours the reaction mixture was cooled to 10 C and 100 ml saturated aqueous ammonium chloride solutionwas added. The reaction mixture was diluted with tert-butyl methyl ether, water and saturated aqueous ammonium chloride solution. The aqueous phase was extracted with tert-butyl methyl ether, the combined organic phases were washed with saturated aqueous ammonium chloride solution and brine and dried over sodium sulfate. The solvent was evaporated and the residue purified by silica gel chromatography using ethyl acetate heptane as eluent. The title compoundswere obtained as yellow liquid (53.7 g) as a 2.6:1 mixture and used for the next reaction without further purification.. A mixture of 2-(4-bromo-5 -fluoro-2-nitro-phenyl)-malonic acid dimethyl ester 2-(2-bromo-5- fluoro-4-nitro-phenyl)-malonic acid dimethyl ester (2.6:1 mixture, 53.7 g, 153 mmol) and 6 Maqueous hydrochloric acid (767 ml) was heated to reflux for 7 hours and then cooled to 5 C. The precipitate was filtered, washed with water and with n-pentane and then coevaporated 3 times with toluene to give 25.9 g of a mixture of the title compounds as white solid. The mother liquor was extracted with ethyl acetate and the combined organic phases dried over sodium sulfate. The solvent was evaporated and the residue triturated with n-pentane and then coevaporated with toluene to give 11.42 g of a mixture of the title compounds as an off-white solid. This material was combined with the first crop to give a total of 37.32 g of the title compounds as a 2.6 :1 mixture which was used for the next reaction without further purification.A suspension of (4-bromo-5-fluoro-2-nitrophenyl)-acetic acid/ (2-bromo-5-fluoro-4-nitro- phenyl)-acetic acid (2.6:1 mixture, 37.3 g, 134 mmol) and iron (30.0 g, 537 mmol) in acetic acid (671 ml) was heated to 100 C for 7 hours and then cooled to room temperature. Remaining elemental iron was removed with a magnetic rod. Ice water (900 ml) was added to the reaction mixture. The precipitate was filtered off, washed four times with water and then suspended in anice-cold aqueous solution of 25 % HC1 (300 ml) and conc. HC1 (50 ml). After stuffing for 10 minutes the precipitate was filtered off and washed four times with water.The precipitate was suspended in a mixture of 1 M aqueous Na2CO3 (400 ml) solution and 0.1 M NaOH (100 ml) and stirred for 40 minutes. The precipitate was filtered off and washed four times with 0.1 M aqueous NaOH, three times with water and once with diisopropylether to givetitle compound as light grey solid (20.5 g). MS ESI (m/z): 228.0/ 230.0 [(M-H)i.1H NMR (DMSO-D6, 400 MHz): (ppm) = 10.47 (bs, 1H), 7.3 1-7.28 (m, 1H), 7.01-6.99 (m, 1H), 3.49 (s, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping