|
With potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In N,N-dimethyl-formamide; at 85℃; for 4h; |
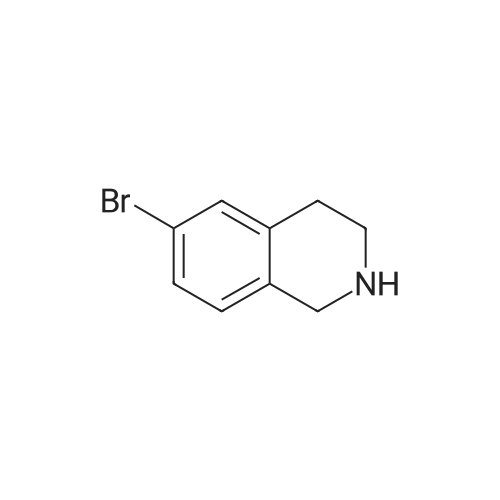
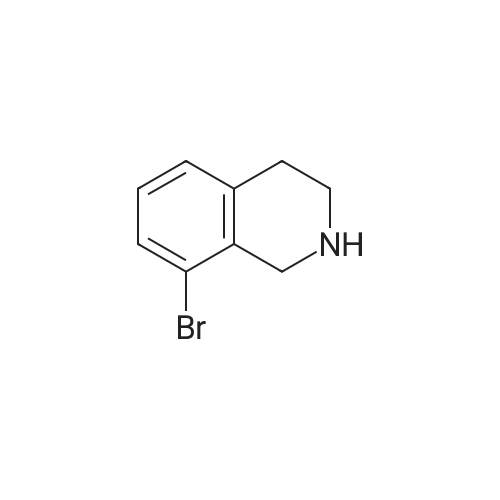
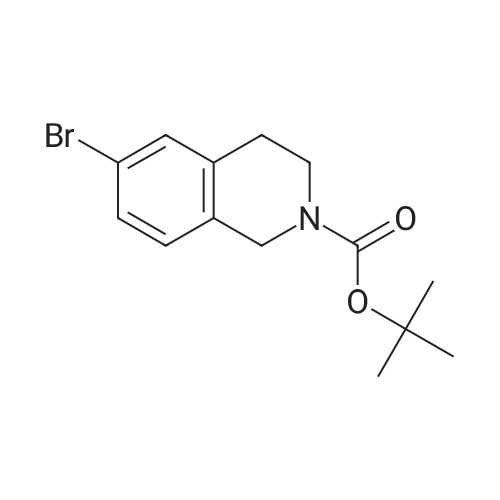
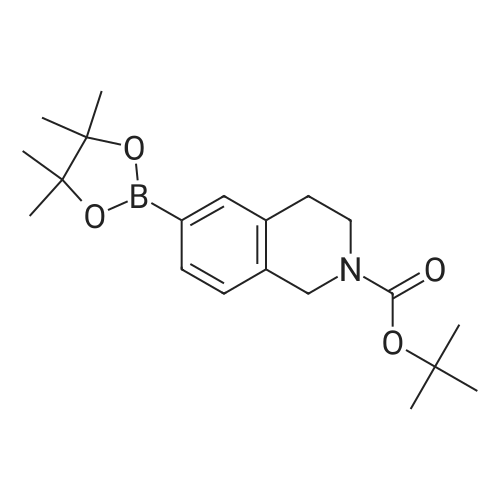
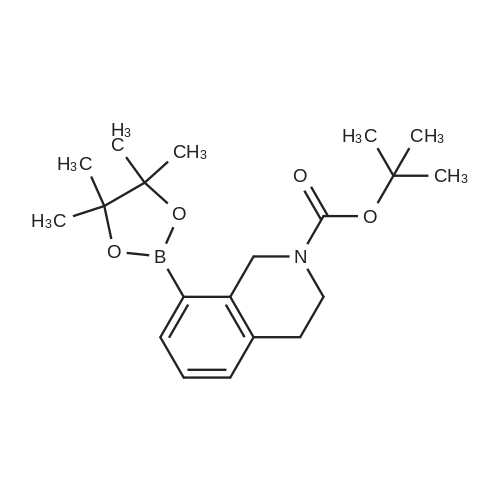
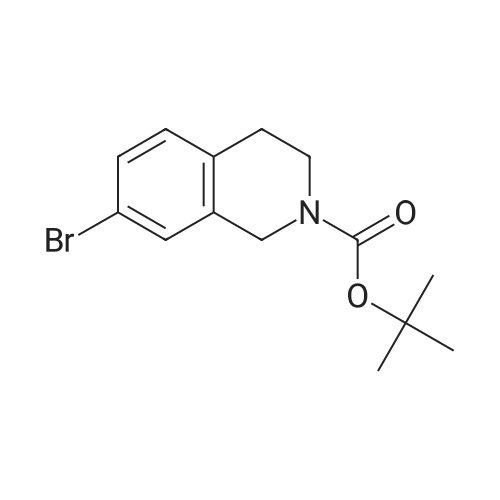
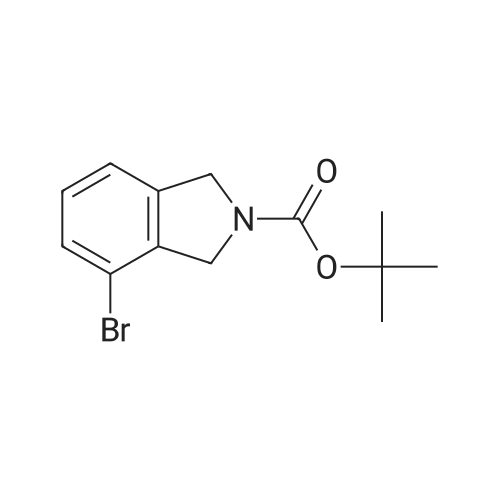
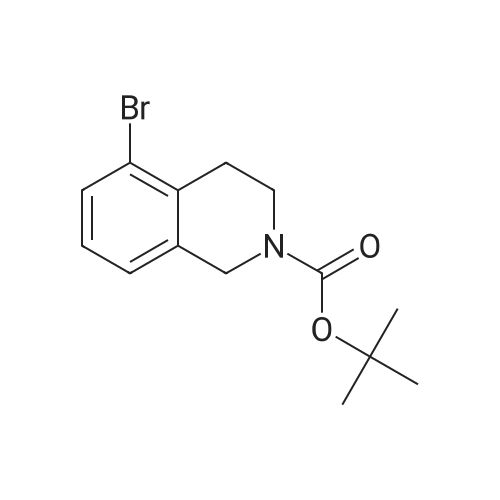
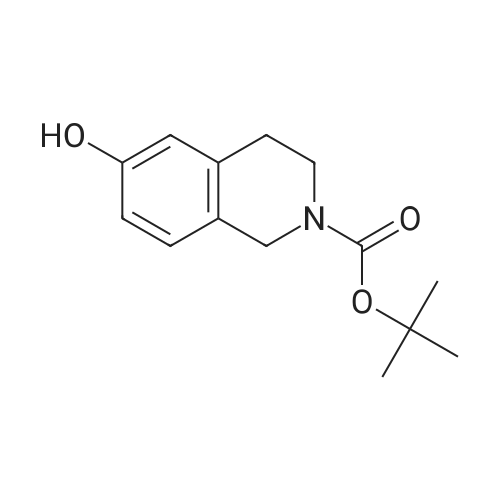
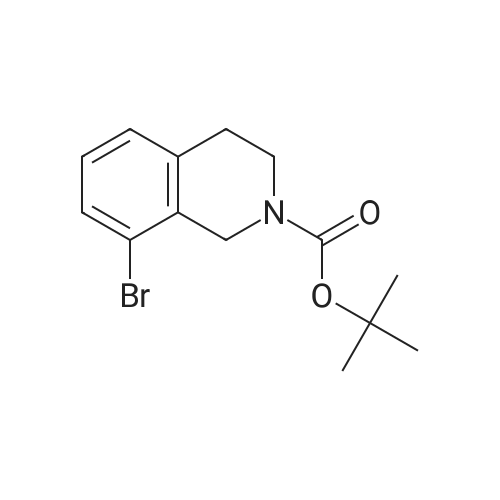
A solution of tert-butyl 6-bromo-3,4-dihydroisoquinoline-2(1H)-carboxylate and <strong>[893566-75-1]tert-butyl 8-bromo-3,4-dihydroisoquinoline-2(1H)-carboxylate</strong> (19.5 mmol), bis(pinacolato)diboron (21.4 mmol) and potassium acetate (61 mmol) in DMF (100 mL) was degassed. To this solution was added PdCl2dppf (1:1 complex with DCM, 0.8 mmol). The reaction mixture was heated at 85 C. for 4 hr and then allowed to cool to room temperature and diluted with EtOAc. The solution was washed with water and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography to give tert-butyl 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate and tert-butyl 8-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate. LCMS: (FA) ES+360 (for each). |
|
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In N,N-dimethyl-formamide; at 81℃; |
tert-Butyl 6-( 4,4,5 ,5-tetramethyl- 1 ,3 ,2-dioxaborolan-2-vD-3 ,4-dihvdroisoquinoline- 2(liD-carboxylate is A 3:1 mixture (0.49 g, 1.6 mmol) of fert-butyl 6-bromo-3,4-dihydroisoquinoline-2(lH)- carboxylate and tert-butyl 8~bromo-3,4-dihydroisoquinoline-2(li/)- carboxylate, bis(pinacolato)diborane (0.45 g, 1.8 mmol), PdCl2 x dppf (0.039 g, 0.048 mmol), potassium acetate (0.48 g, 4.8 mmol) and DMF (8.0 mL) was heated at 81 C ' WeligKtyThe~slve1alphat^30 twice with EtOAc. The organic phase was dried, filtered and concentrated. Column chromatography with EtO Ac-heptanes (1:10 through 1:4) gave 0.24 g of a 4:1 mixture of EPO <DP n="25"/>the title product and ter/-butyl 8-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-3,4- dihydroisoquinoline-2(lH)-carboxylate.1H NMR (CDCl3) delta 7.62 (d, IH), 7.60 (s, IH), 7.13 (d, IH), 4.59 (s, 2H), 3.64 (t, 2H), 2. .85 (t, 2H), 1.50 (s, 9H) and 1.35 (s, 12H) ppm (6-isomer). 1H NMR (CDCl3) delta 7.69 (d, IH), 7.24-7.14 (m's, 2H), 4.88 (s, 2H), 3.64 (t, 2H), 2.85 (t, 2H), 1.50 (s, 9H) and 1.35 (s, 12H) ppm (8-isomef). |
|
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In N,N-dimethyl-formamide; at 81℃; |
A 3:1 mixture (0.49 g, 1.6 mmol) of tert-mty 6-bromo-3,4-dihydroisoquinoline-2(lH)- carboxylate and fert-butyl 8-bromo-3,4-dihydroisoquinoline-2(lH)- carboxylate, bis(pinacolato)diborane (0.45 g, 1.8 mmol), PdCl2 x dppf (0.039 g, 0.048 mmol), potassium acetate (0.48 g, 4.8 mmol) and DMF (8.0 mL) was heated at 81 0C overnight. The solvent was evaporated, the residue taken up in water-brine and washed twice with EtOAc. The organic phase was dried, filtered and concentrated. Column chromatography with EtOAc-heptanes (1:10 through 1:4) gave 0.24 g of a 4:1 mixture of the title product and tert-butyl 8-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-3,4- dihydroisoquinoline-2( lH)-carboxylate.1H NMR (CDCl3) delta 7.62 (d, IH), 7.60 (s, IH), 7.13 (d, IH), 4.59 (s, 2H), 3.64 (t, 2H)5 2.85 (t, 2H), 1.50 (s, 9H) and 1.35 (s, 12H) ppm (6-isomer). 1H NMR (CDCl3) delta 7.69 (d, IH), 7.24-7.14 (m, 2H)5 4.88 (s, 2H), 3.64 (t, 2H), 2.85 (t, 2H), 1.50 (s, 9H) and 1.35 (s, 12H) ppm (8-isomer). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping