| 56% |
With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; XPhos; In 1,4-dioxane; at 100℃; for 6.0h;Inert atmosphere; |
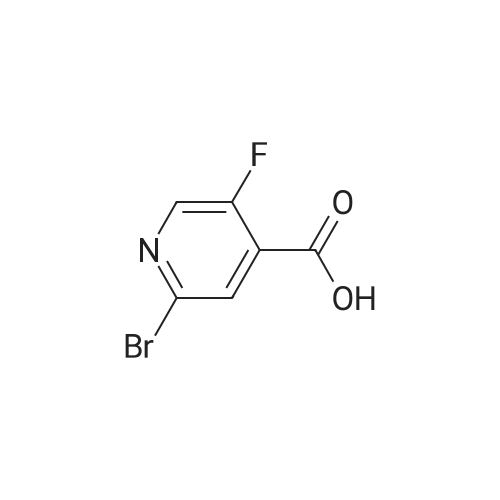
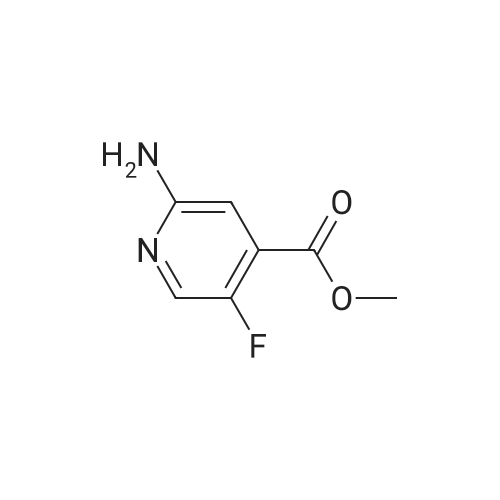
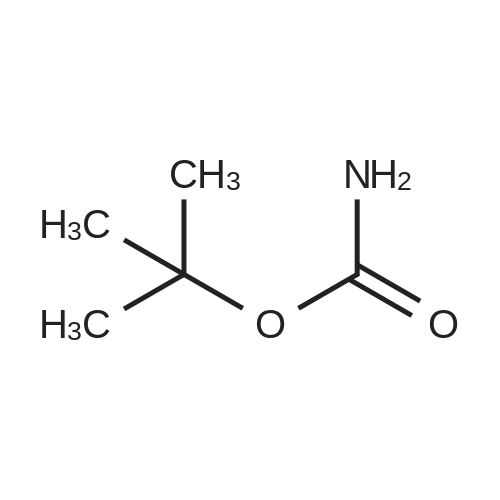
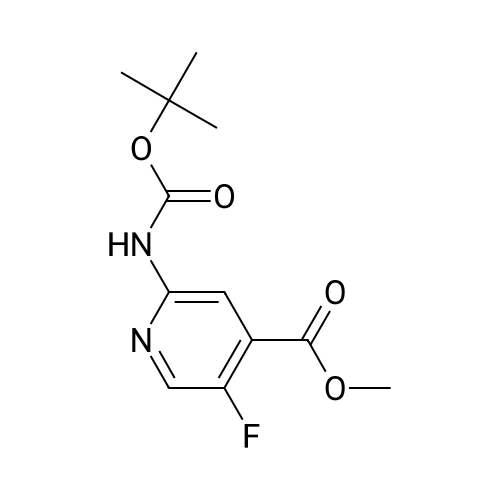
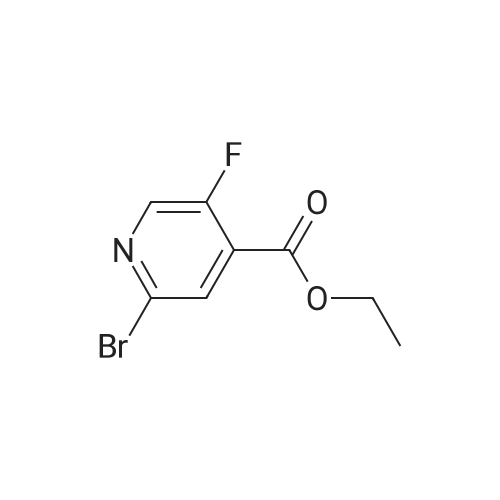
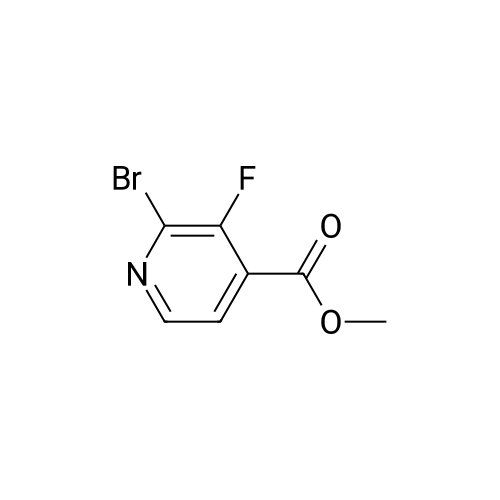
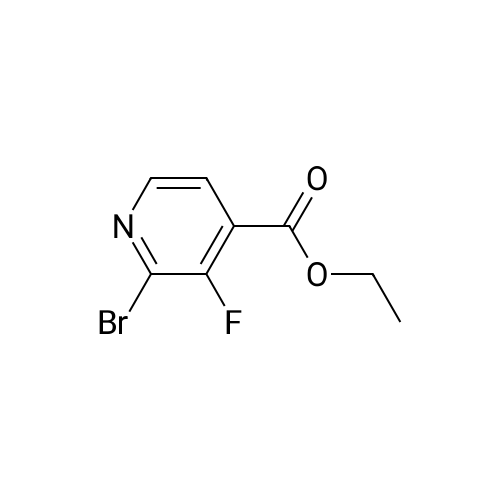
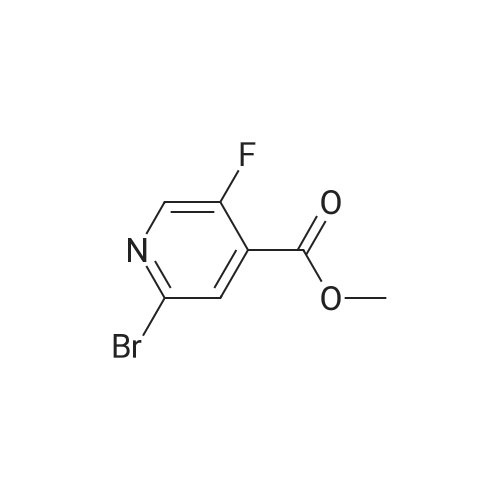
A mixture of <strong>[885588-14-7]methyl 2-bromo-5-fluoropyridine-4-carboxylate</strong> (6.64 g, 28.4 mmol), tert- Butyl carbamate (4.0 g, 34.1 mmol), cesium carbonate (13.0 g, 39.8 mmol), X-phos (657 mg, 1.1 mmol) and Pd(dba)2 (520 mg, 0.9 mmol) in 1,4-dioxane (100 mL) was purged with nitrogen and then stirred at 100 C under nitrogen for 6 h. The mixture was cooled to RT, diluted with water (100 mL), and extracted with EA (150 mL x 2). The combined organic phase was washed with brine, dried over MgSO4, and concentrated. The residue was chromatographed, eluting with 0- 30% EA in hexane to give methyl 2-((tert-butoxycarbonyl)amino)-5-fluoropyridine-4- carboxylate as a white solid (4.5 g, 56%). MS (ESI+) m/z 271.1 (M+H)+, retention time: 1.81 min. (Method A). 1H NMR (400 MHz, CDCl3) δ 1.52 (s, 9H), 3.96 (s, 3H), 8.24 (s, 1H), 8.40 (d, 1H), 9.00 (s, 1H). |
| 56% |
With caesium carbonate; bis(dibenzylideneacetone)-palladium(0); XPhos; In 1,4-dioxane; at 100℃; for 6.0h;Inert atmosphere; |
A mixture of <strong>[885588-14-7]methyl 2-bromo-5-fluoropyridine-4-carboxylate</strong> (6.64 g, 28.4 mmol), tert-Butyl carbamate (4.0 g, 34.1 mmol), cesium carbonate (13.0 g, 39.8 mmol), X-phos (657 mg, 1.1 mmol) and Pd(dba)2 (520 mg, 0.9 mmol) in 1,4-dioxane (100 mL) was purged with nitrogen and then stirred at 100 C under nitrogen for 6 h. The mixture was cooled to RT, diluted with water (100 mL), and extracted with EA (150 mL x 2). The combined organic phase was washed with brine, dried over MgS04, and concentrated. The residue was chromatographed, eluting with 0-30% EA in hexane to give methyl 2-((tert-butoxycarbonyl) amino)-5-fluoropyridine-4-carboxylate as a white solid (4.5 g, 56%). MS (ESI+) m/z 271.1 (M+H)+, retention time: 1.81 min. (Method A). 'H NMR (400 MHz, CDCl3) d 1.52 (s, 9H), 3.96 (s, 3H), 8.24 (s, 1H), 8.40 (d, 1H), 9.00 (s, 1H). |
| 55.7% |
With caesium carbonate;tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; In 1,4-dioxane; at 100℃; for 5.5h;Inert atmosphere; |
b) methyl 2-(tert-butoxycarbonylamino)-5-fluoroisonicotinate To an nitrogen purged suspension of <strong>[885588-14-7]methyl 2-bromo-5-fluoroisonicotinate</strong> (2.8 g, 12 mmol) in dioxane (55 ml) is added successively tert-butyl carbamate (1.68 g, 14.4 mmol), tris(dibenzylidene-acetone)dipalladium(0) (219 mg, 239 umol), 4,5-bis(diphenylphos-phino)-9,9-dimethylxanthene (277 mg, 479 mmol) and cesium carbonate (5.46 g, 16.8 mmol). The mixture is then stirred for 5.5 hrs at 100 C. under nitrogen atmosphere. After 5 min at 100 C. the redbrown suspension has turned green. The mixture is diluted with ethyl acetate, washed twice with water, once with brine, dried with magnesium sulfate and the solvent is removed in vacuo. The product is isolated by chromatography of the residue (3.85 g) on a 70 g Silicycle silica cartridge using a heptane/ethyl acetate 10-40% gradient affording methyl 2-(tert-butoxycarbonylamino)-5-fluoroisonicotinate (1.8 g, 55.7%) as a light yellow solid. MS: m/z=271.2 (M+H+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping