| 82% |
|
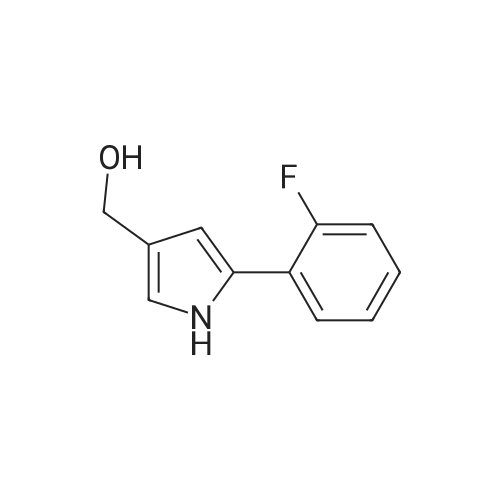
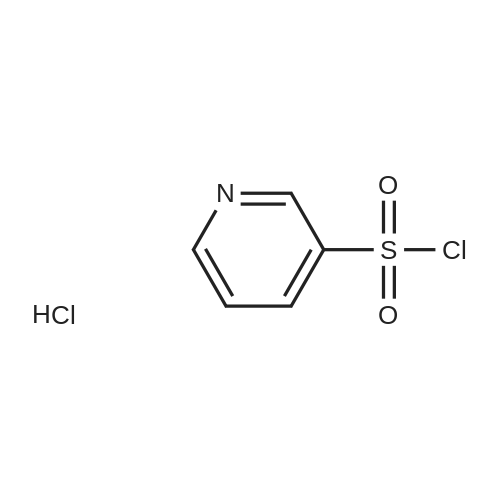
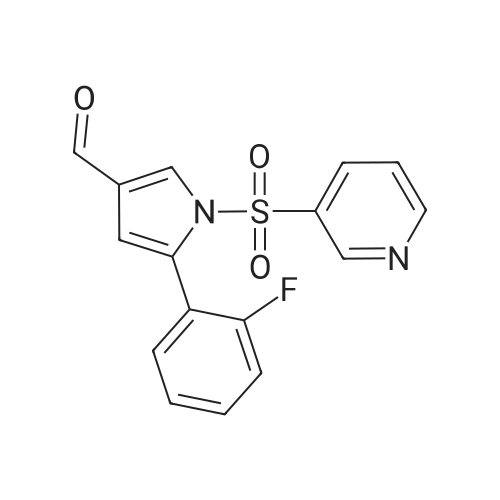
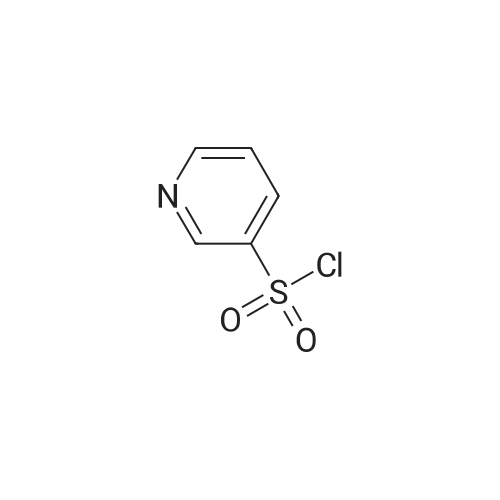
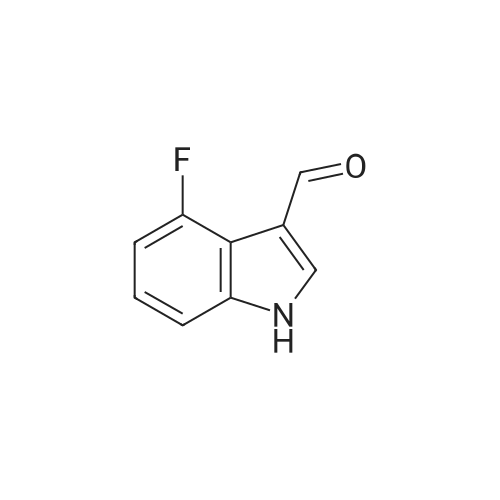
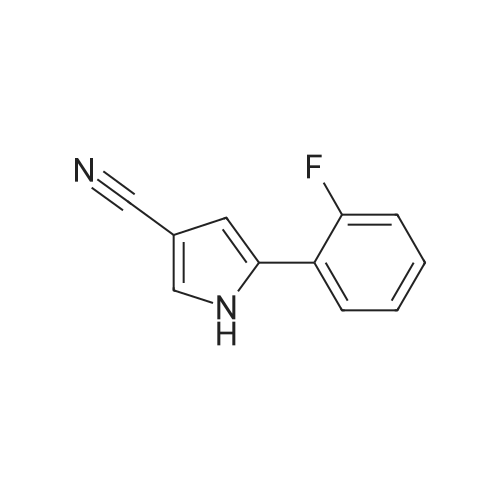
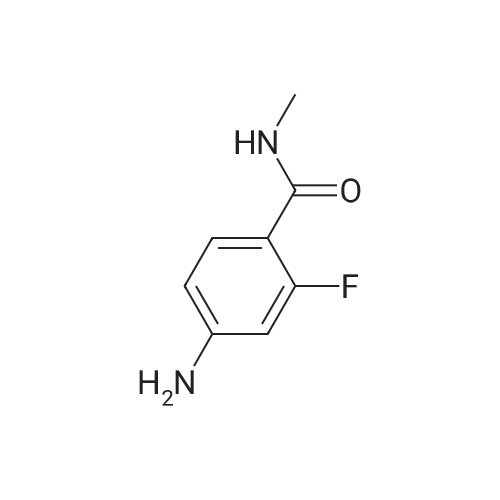
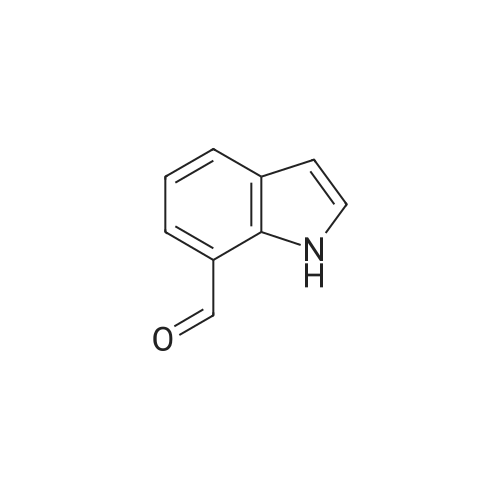
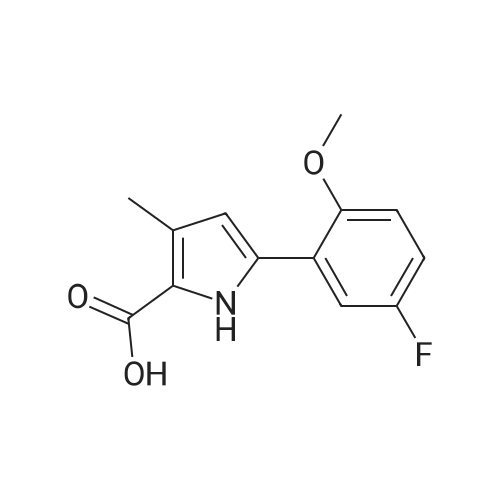
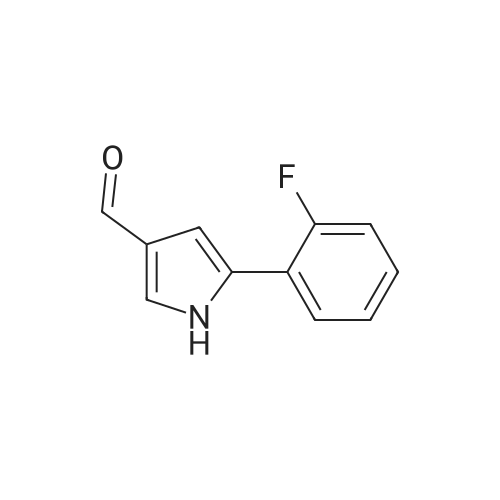
Reference Example 245 5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde To a solution (96 mL) of <strong>[881674-56-2]5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde</strong> (475 mg) in tetrahydrofuran was added sodium hydride (60percent in oil, 503 mg) at room temperature and the mixture was stirred for 30 min. 15-Crown-5 (2.77 g) was added dropwise and the mixture was stirred for 30 min, pyridine-3-sulfonyl chloride hydrochloride (1.35 g) was added, and the mixture was further stirred for 3 hr. Saturated brine was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The extract was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate=7:3?2:3), and crystallized from diisopropyl ether-ethyl acetate (4:1) to give the title compound as colorless crystals (yield 680 mg, 82percent). 1H-NMR (CDCl3)delta: 6.68 (1H, d, J=1.8 Hz), 6.99-7.05 (1H, m), 7.16-7.19 (2H, m), 7.35-7.39 (1H, m), 7.45-7.51 (1H, m), 7.69-7.73 (1H, m), 8.14 (1H, d, J=1.8 Hz), 8.58-8.59 (1H, m), 8.81-8.83 (1H, m), 9.91 (1H, s). |
| 78% |
|
A total of 0.5g, 4-dimethylaminopyridine 90mg, 5ml acetonitrile, take N, N-diisopropylethylamine1.5g with a small amount of acetonitrile dissolved in the above reaction bottle, take pyridine-3-sulfonyl chloride hydrochloride 1.1g, with 5mlAcetonitrile dissolved into the reaction flask, heated to 45 ° C stirring reaction 5h after cooling to room temperature, adding 5ml of water,The pH was adjusted to 5 with 0.5 N hydrochloric acid solution, and 20 ml of water was slowly added dropwise (at this time, a large amount of white solid precipitated)Stirring at room temperature for 0.5h, cooling to below 10 , stirring 1h, filtration, filter cake with a small amount of acetonitrile and water mixtureWashing, 50 ° C drying was V total of 0.69g, yield 78percent. |
|
In tetrahydrofuran; |
Reference Example 245 5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde To a solution (96 mL) of <strong>[881674-56-2]5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde</strong> (475 mg) in tetrahydrofuran was added sodium hydride (60percent in oil, 503 mg) at room temperature and the mixture was stirred for 30 min. 15-Crown-5 (2.77 g) was added dropwise and the mixture was stirred for 30 min, pyridine-3-sulfonyl chloride hydrochloride (1.35 g) was added, and the mixture was further stirred for 3 hr. Saturated brine was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The extract was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate=7:3-->2:3), and crystallized from diisopropyl ether-ethyl acetate (4:1) to give the title compound as colorless crystals (yield 680 mg, 82percent). 1H-NMR (CDCl3)delta: 6.68 (1H, d, J=1.8 Hz), 6.99-7.05 (1H, m), 7.16-7.19 (2H, m), 7.35-7.39 (1H, m), 7.45-7.51 (1H, m), 7.69-7.73 (1H, m), 8.14 (1H, d, J=1.8 Hz), 8.58-8.59 (1H, m), 8.81-8.83 (1H, m), 9.91 (1H, s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping