| 80% |
With hydrogenchloride; In ethanol; for 8h;Heating / reflux; |
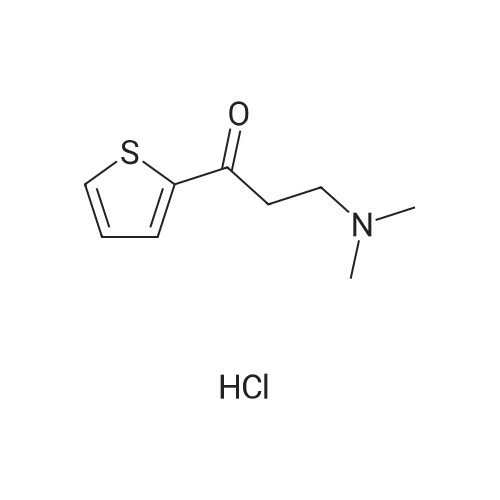
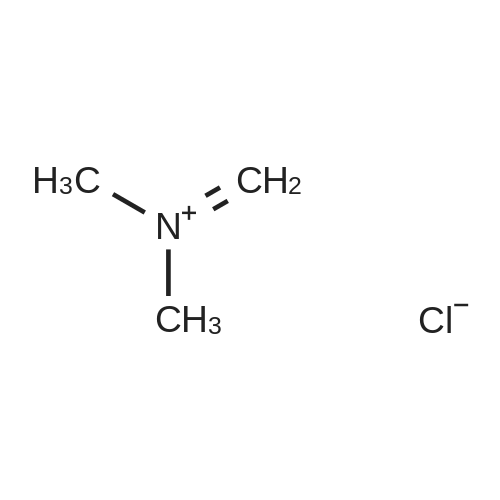
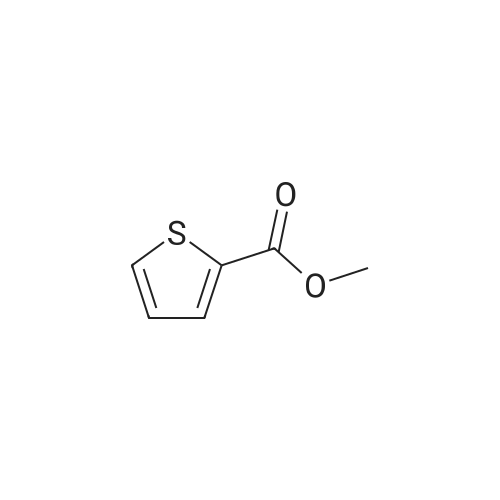
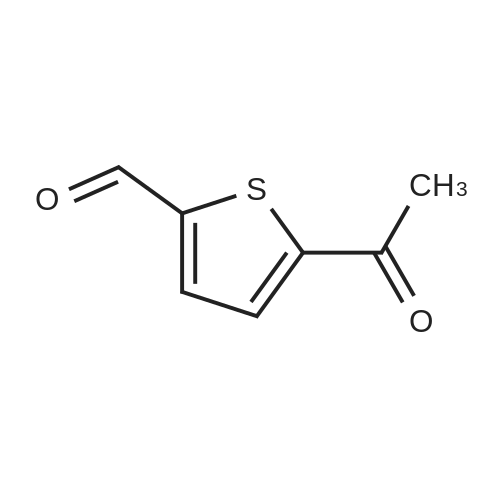
A mixture of 2-acetylthiophene (100 g, 0.79 mol), dimethylamine hydrochloride (84.3 g, 1.03 mol), Para formaldehyde (31.4 g, 0.35 mol) and 35% w/w hydrochloric acid (2 g, 0.02 mol) in ethanol (130 ml) was refluxed for 8 hours. The mixture was then cooled to about 20-25C and diluted with ethanol (160 ml) and acetone (745 ml). The mixture was stirred for 2 hours and the solid was collected by filtration to yield 140 g (80%) of 3-dimethylamino-1-(2-thienyl)-1-propanone hydrochloride as a colorless crystalline solid |
| 76% |
With hydrogenchloride; In water; isopropyl alcohol; at 100℃; for 6h; |
2-acetyl thiophene (2-acetylthiophene were dissolved in; (50 isopropyl alcohol); IPA 10.0 g, 80mmol), dimethylamine hydrochloride (dimethylamine hydrochloride; 16.14 g, 198mmol, 2.5 eq.), paraformaldehyde (paraformaldehyde; 6.00 g, 199.8 mmol, 2.4 perAmount) and 37% HCl (3.9 , 39.6 mmol, 0.5 eq.) The mixture100 inAnd heated for 16 hours.The reaction mixture was cooled to room temperature and the solid was filtered and washed with IPA (50 mL).The white solid was dried in vacuo to give the product (13.3 g, 76% yield). |
| 73% |
With hydrogenchloride; In ethanol; acetone; for 2h;Reflux; |
2-acetylthiophene (DX-A 01, 63.1 g, 0.50 mol), dimethylamine hydrochloride (53.0 g, 0.65 mol)Paraformaldehyde (19.8 g, 0.22 mol)And 1 N hydrochloric acid (1 mL) in ethanol (80 mL) was heated and refluxed for 2 hours. This solution was diluted with ethanol (100 mL) and acetone (500 mL)The resulting mixed solution was cooled to 0 C. and stirred for 1 hour more.Next, the obtained crystals were separated by filtration under reduced pressure,And washed with ethanol. The washed solid was heated at 60 C. for 5 hours,Dried under reduced pressure,2-thienyl 2-dimethylaminoethyl ketone hydrochloride(DXA 02 · HCl, 75.0 g, 73%). |
| 73% |
With hydrogenchloride; In ethanol; water; for 2h;Reflux; |
2-acetylthiophene (DX-A 01, 63.1 g, 0.50 mol),Dimethylamine hydrochloride (53.0 g, 0.65 mol),Paraformaldehyde (19.8 g, 0.22 mol)And 1 N hydrochloric acid (1 mL) in ethanol (80 mL) was heated,And refluxed for 2 hours.This solution was diluted with ethanol (100 mL) and acetone (500 mL)The obtained mixed solution was cooled to 0 C.,The mixture was further stirred for 1 hour or more.Next, the obtained crystals were separated by filtration under reduced pressure,And washed with ethanol.The washed solid was heated at 60 C. for 5 hours,Dried under reduced pressure,2-thienyl 2-dimethylaminoethyl ketone hydrochloride(DX-A 02 .HCl, 75.0 g, 73%). |
| 69% |
With hydrogenchloride; In isopropyl alcohol; at 70℃; for 18h; |
To a solution ofTo 37% HCl (0.60 ml,19.8mmol, 0.5Equiv) in 2-propanol (73 mL)middleThe stirred solutionPressSequential addition of paraformaldehyde(3.00 g,95.1 mmol, 2.4 equiv.), DimethylamineHydrochloride(8.07 g, 99.0 mmol, 2.5 eq.), As well2-Acetylthiophene (5.0 g,39.6 mmol). The cloudy suspension was heated to 70 & lt; 0 & gt; C and gradually turned to a clear homogeneous mixture. Lt; 0 & gt; CAfter about 18 hours a white precipitate had formed. The reaction mixture was cooled to room temperature and the white solid was takenFiltered and washed with ice-cold ethanol (2 x 30 mL). The white solid was dried at 50 & lt; 0 & gt; C in a vacuum ovenDeg.] C for 12 hours to give 6.0 g (69%) of 1 as a white solid |
| 60.4% |
In isopropyl alcohol; for 3h;Heating / reflux; |
252.4 g (2.0 mol) 2-acetylthiophene are dissolved in 160 ml isopropanol and added with stirring to 60.1 g (2.0 mol) of paraformaldehyde. Then, 163.1 g (2.0 mol) dimethylamine-hydrochloride are added and the mixture is rinsed with another 100 ml isopropanol. The thick suspension obtained is refluxed for about 3 hours. The suspension is diluted with another 400 ml isopropanol and cooled to about 15 C., suction filtered and washed with 400 ml isopropanol in batches; then it is dried overnight at 60 C. in the vacuum drying cupboard, yield 265.6 g (60.4% of theory), purity >98% according to NMR. |
|
With hydrogenchloride; In isopropyl alcohol; at 75 - 80℃; for 6h; |
Examples Example-1: Preparation of 3-(dimethylamino)-l-(thiophen-2-yl) propan-1-one hydrochlorideAdded 3.8 Kgs. of hydrochloric acid to a solution of 100 Kgs. of 2-acetyl thiophene, 81.5 Kgs. of dimethylamine hydrochloride, 35.4 Kgs. parafomaldehyde and 250 liters of isopropyl alcohol. Heated the reaction mixture to 75-80C. Stirred the reaction mixture for 6 hours at 75-80C. Cooled the reaction mixture to 0-5C. Stirred the reaction mixture for 2 hours at 0-5C. Filtered the solid and washed with isopropyl alcohol.Yield : 151 KgsM.R: 174-176CExample-2:Purification of 3-(dimethyIamino)-l-(thiophen-2-yl) propan-1-one hydrochloride:Added 1500 liters of isopropyl alcohol and 45 liters of water to 151 Kgs of 3- (dimethylamino)-l-(thiophen-2-yl) propan-1-one hydrochloride. Stirred the reaction mixture for 15 minutes at 25-3O0C. Heated the reaction mixture to reflux. Stirred the reaction mixture for 2 hours at reflux. Cooled the reaction mixture slowly to 25-30C. Stirred the reaction mixture for 4 hours at 25-3O0C. Filtered the solid and washed with isopropyl alcohol. Dried the material at 25-300C for 2 hours followed by drying at 50- 550C for 6 hours to get the pure title compound. Yield: 144 Kgs. M.R: 185-1900C. <n="16"/>Example-3:Purification of 3-(dimethylamino)-l-(thiophen-2-yl) propan-1-one hydrochloride:Added 1500 liters of acetone and 45 liters of water to 151 Kgs of 3-(dimethylamino)-l- (thiophen-2-yl) propan-1-one hydrochloride. Stirred the reaction mixture for 15 minutes at 25-30C. Heated the reaction mixture to reflux. Stirred the reaction mixture for 2 hours at reflux. Cooled the reaction mixture slowly to 25-30C. Stirred the reaction mixture for 4 hours at 25-300C. Filtered the solid and washed with acetone. Dried the material at 25- 3O0C for 2 hours followed by drying at50-55C for 6 hours to get the pure title compound. Yield: 142 Kgs. M.R: 185-1900C. |
|
With hydrogenchloride; In water; isopropyl alcohol; at 0℃; for 18h;Heating / reflux; |
a) Preparation of 3-dimethyiammo~l~(2~thieRyE)-l-propanaoiie hydrochloride:; A mixture of 2-acetyithiorhohene (100 g), dimethylaraine hydrochloride (81.21 g), paraformaldehyde (35.36 g) and concentrated hydrochloric acid (3.7 g) in isopropyl alcohol (240 mL) was stirred under reflux for 12 h. The mixture was cooled to 0~5C and stirred at the same temperature for 6 h. The solid was filtered, washed with cold (5-1O0C) isopropyl alcohol (100 mL), and dried under vacuum at 45-500C for 12 h to obtain the title compound as a white solid.Yield; 135 g |
|
With hydrogenchloride; In ethanol; water; for 4 - 5h;Heating / reflux;Product distribution / selectivity; |
Example 1; (S)- 3-(dimethylamino)-l-(2-thienyl)-l-propanol (5): <n="11"/>lambda mixture of 2-acetyl thiophene (250 g, 1.98 mol), dimethyl amine hydrochloride (21 O g, 2.57 mole), paraformaldehyde (78.44 g, 0.871 mole) and concentrated HCl (3.97 ml) in ethanol (317 ml) was refluxed for 4 h. The reaction mixture was cooled to the room temperature and water (700 ml) was added, followed by addition of methanol (1.6 L) at room temperature. The reaction mixture was cooled to 5-10 C and basified with aqueous sodium hydroxide solution to adjust the pH 7-8. Then sodium borohydride (81 g, 2.18 mole) was added in small lots at 5-10 C. After completion of addition of sodium borohydride, reaction mixture was stirred for 30 minutes at 5-10 C. Then it was stirred at room temperature for 12-15 h. The reaction mixture was concentrated to obtain oily residue. The residue was treated with ethyl acetate (3.0 L) and washed with water (3x500 ml) and brine solution (500 ml). The ethyl acetate layer was separated, dried over sodium sulphate, and concentrated to obtain oil which solidified on standing to white solid (210 g). This solid was taken in methyl tert butyl ether (3.15 L) and heated to 45-50 C and a solution of (S)-(+)-Mandelic acid (86.27 g. 0.567 mole) in ethanol (250 ml) was added in a dropwise manner. The reaction mixture was stirred for 45 minutes at 45-50 C and cooled to room temperature. The solid obtained was filtered and washed with methyl tert butyl ether to obtain Mandelate salt (112 g). This salt was crystallized using acetone to get pure Mandelate salt (96 g). The Mandelate salt was basified with sodium hydroxide followed by extraction with dichloromethane to obtain (S)- 3-(dimethylamino)-l-(2- thienyl)-l-propanol as oil which solidified on standing. Yield 50g.Example 2(S)- 3-(dimethylamino)-l-(2-thienyl)-l-propanol (5):lambda mixture of 2-acetyl thiophene (25Og, 1.98 mole), dimethyl amine hydrochloride (21 O g, 2.57 mole), paraformaldehyde (78.44 g, 0.871 mole) and concentrated HCl (3.97 ml) in ethanol (317 ml) was refluxed for 4 h. The reaction mixture was cooled to the room temperature and water (700 ml) was added followed by addition of methanol (1.6 L) at room temperature. The reaction mixture was cooled to 5-10 C and basified with aqueous sodium hydroxide solution to adjust pH 7-8. Then sodium borohydride (81 g, 2.18 mole) was added in small lots at 5-10 C. After completion of addition of sodium borohydride, reaction mixture was stirred for 30 minutes at 5-10 C. Then it was stirred at room <n="12"/>temperature for 12-15 h. After completion of reaction, acetone (240 ml) was added to quench the excess borohydride. The reaction mixture was concentrated to obtain oily residue. To this residue dilute aqueous HCl solution (250 ml cone HCl in 250 ml water) was added to form the hydrochloride salt. This aqueous solution was washed with toluene (2x200 ml) to remove organic impurities. Washed aqueous solution was basified with aqueous alkali solution to pH 10-11. This alkaline solution was then extracted with methyl tert butyl ether (3x750 ml). The organic layer was then washed with water and dried over sodium sulphate. The organic layer was heated to 45-50 C and a solution of (S)-(+)-Mandelic acid (86.27 g, 0.567 mole) in ethanol (250 ml.) was added in a dropwise manner. The reaction mixture was stirred for 45 minutes at 45-50 C and cooled to room temperature. The solid obtained was filtered and washed with acetone to obtain Mandelate salt. This salt was leached in acetone at reflux temperature to get pure Mandelate salt (98 g). The Mandelate salt was basified with sodium hydroxide followed by extraction with dichloromethane to obtain (S)- 3-(dimethylamino)-l-(2-thienyl)-l- propanol as oil which solidified on standing. Yield 52 g.Example 3(S)- 3-(dimethylamino)-l-(2-thienyl)-l-propanol (5):A mixture of 2-acetyl thiophene (100 g, 0.793 mole), dimethyl amine hydrochloride (81.5 g, 1 mole), paraformaldehyde (35.5 g, 0.394 mole) and concentrated HCl (4.0 ml) in ethanol (250 ml) was refluxed for 5 h. The reaction mixture was cooled to room temperature and diluted with ethanol (250 ml). The reaction mixture was cooled to 5-10 C and basified with ethanolic sodium hydroxide solution (32 g NaOH in 450 ml ethanol) to pH 8-10. The sodium borohydride (35 g, 0.92mol) was added portionwise into the reaction mixture at 5-100C for 30 minutes. The reaction mixture was stirred at room temperature for 6 h. Then glacial acetic acid (165 ml) was added into it to adjust pH 5-6 to get a white solid which was filtered and washed with ethanol (300 ml). The filtrate was basified with 20% aqueous NaOH (140 ml) to pH 7.5-8 and concentrated to obtain oily residue (112 g). The residue was dissolved in acetone (700 ml) at 45-500C and a solution of (S)-(+)-Mandelic acid (47 g, 0.31 mole) in acetone (100 ml) was added in a drop wise manner at 45-500C. The reaction mixture was stirred for 45 min at 45-50 C and was <n="13"/>further cooled to room ... |
|
With hydrogenchloride; In water; isopropyl alcohol; for 6h;Heating / reflux;Product distribution / selectivity; |
Example 4; (S)- 3-(dimcthylamino)-l-(2-thienyl)-l-propanol (5):A mixture of 2-acetyl thiophene (100 g, 0.793 mole), dimethylamine hydrochloride (81.5 g, 1 mole), paraformaldehyde (35.5 g, 0.394 mole) and concentrated HCl (4.0 ml) in isopropanol (IPA) (250 ml) was refluxed for 6 h. The reaction mixture was cooled to room temperature and IPA was removed by distillation. Water (600 ml) was added to the residue. A solution of sodium borohydride (15 g) in 10% aqueous sodium hydroxide solution (41 g, NaOH in 410 ml water) was slowly added to the reaction mixture at 30-350C. The reaction mixture was stirred at room temperature for 6 h. Then aqueous HCl (Cone HCl 150 ml in 300 ml water) was added to get a clear solution. The reaction' mass was then washed with toluene (2x300 ml) to remove organic impurities. The reaction mass was again basified by adding sodium hydroxide solution (75 g NaOH in 150 ml water). This basic solution was extracted with methyl tert butyl ether (3x300 ml). The ethereal layer was washed with water and dried over sodium sulphate. This organic layer was heated to 50-550C and a solution of (S)-(+)-Mandelic acid (44 g) in ethanol (44 ml) was added. The reaction mass was heated at this temperature for 1 h and cooled to rt and filtered to get chiral Mandelate salt. This crude salt was taken in acetone (900 ml) and the mixture was heated at reflux temperature for 3 h. Then reaction mixture was cooled and filtered to get the pure Mandelate salt of the compound 5 (84 g). The Mandelate salt was basified with sodium hydroxide and extracted with dichloromethane to obtain (S)- 3- (dimethylamino)-l-(2-thienyl)-l-propanol as oil, which solidified on standing. Yield 42 g. The mother liquor containing the unwanted isomer (8) was concentrated, basified and extracted with ethyl acetate to get the oily residue which solidified on standing. <n="14"/>Example 5(S)- 3-(dimethylamino)-l-(2-thienyl)-l-propanol (5)A mixture of 2-acetyl thiophene (100 g, 0.793 mole), dimethyl amine hydrochloride (81.5 g, 1 mole), paraformaldehyde (35.5 g, 0.394 mole) and concentrated HCl (4.0 ml) in isopropanol (250 ml) was refluxed for 6 h. The reaction mixture was cooled to 5-100C and filtered and washed with IPA to get a white colored solid. This solid (150 g) was dissolved in water (600 ml). A solution of sodium borohydride (12.5 g, 0.342 mole) in 10% aqueous sodium hydroxide solution (41 g, NaOH in 410 mL water) was slowly added to the reaction mixture at 30-350C. The reaction mixture was stirred at room temperature for 6 h. Then aqueous HCl (150 ml cone HCl in 300 ml water) was added to get a clear solution. The reaction mass was then washed with toluene (2x300 ml) to remove organic impurities. The reaction mass was then basified using sodium hydroxide solution (75 g NaOH in 150 ml water) and then extracted using methyl tert butyl ether. (3x300 ml). The ethereal layer was washed with water and dried over sodium sulphate. This organic layer was heated to 50-550C and a solution of (S)-(+)-Mandelic acid (44 g, 0.289 mole) in ethanol (44 ml) was added. The reaction mass was heated at this temperature for 1 h, cooled to rt and filtered to get crude Mandelate salt. This crude salt was taken in acetone (900 ml) and the mixture was heated at reflux temperature for 3 h. Then reaction mixture was cooled and filtered to get the pure Mandelate salt of the compound 5 (84 g). The Mandelate salt was basified with sodium hydroxide and extracted with dichloromethane to obtain (S)- 3-(dimethylamino)-l-(2-thienyl)-l- propanol as oil which solidified on standing. Yield 42 g.The mother liquor containing the unwanted isomer (8) was concentrated, basified and extracted with ethyl acetate to get the oily residue which solidified on standing. |
|
With hydrogenchloride; In water; isopropyl alcohol; for 5h;Heating / reflux; |
A mixture of 2-acetylthiophene (800 g), N,N-dimethylamine hydrochloride (652.2 g), paraformaldehyde (282.1 g), concentrated hydrochloric acid (30.3 ml) and isopropyl alcohol (2.4 It) was refluxed for 5 hours followed by cooling to 20-25 0C. The solid thus obtained, was filtered, washed with isopropyl alcohol (800 ml) and dried to obtain 1.194 kg of title compound having purity 99.29% by HPLC. |
|
|
Example-1: Preparation of 3-(N, N-Dimethylamino)-l-(2-thienyl) propan-1-one hydrochlorideCharge Isopropanol (250 ml) to the flask at 25-35C. Charge Dimethyl amine hydrochloride (77.5 g) to the flask followed by Cone. HCl (4.0 ml) under stirring. Charge Paraformaldehyde (33.33 g) to the flask under stirring. Stir the reaction mass for 30 mins. Add 2- Acetyl thiophene (100.0 g) to the flask. Heat the reaction mixture and stir the reaction mixture at 70-750C. After the completion of the reaction; cool the reaction mass. Filter the content. Wash the wet cake with Isopropanol. Suck dry the material. Dry the material in hot air oven. |
|
With hydrogenchloride; In isopropyl alcohol; for 4h;Heating / reflux; |
Example 1 Preparation of AT-ONE A mixture of 50 g of 2-acetylthiophene (containing 0.56% 3-acetylthiophene), 42 g of dimethylamine hydrochloride, 18 g of paraformaldhyde, and 2 g of HCl [32%] in 125 ml IPA were heated to reflux for 4 hours. The mixture was cooled to 0 C., and the resulting solid was collected by filtration, washed with ethanol (125 ml×2), and used in the next step without further action. |
|
With hydrogenchloride; In water; isopropyl alcohol; for 12h;Reflux; |
Example 1Preparation of Duloxetine Maleatea) Preparation of 3-dimethylamino-1-(2-thienyl)-1-propanaone hydrochlorideA mixture of 2-acetylthiophene (100 g), dimethylamine hydrochloride (81.21 g), paraformaldehyde (35.36 g) and concentrated hydrochloric acid (3.7 g) in isopropyl alcohol (240 mL) was stirred under reflux for 12 h. The mixture was cooled to 0-5 C. and stirred at the same temperature for 6 h. The solid was filtered, washed with cold (5-10 C.) isopropyl alcohol (100 mL), and dried under vacuum at 45-50 C. for 12 h to obtain the title compound as a white solid.Yield: 135 g |
|
With hydrogenchloride; In ethanol; for 3h;Reflux; |
General procedure: A mixture of the methylketone (0.20 mol), dimethylamine hydrochloride(0.20 mol), paraformaldehyde (0.25 mol), and concentrated HCl(0.50 mL), in 30 mL of 95% ethanol, was refluxed for 3 h. Aftercooling, acetone (150 mL) was added and the mixture was lef tovernight in the refrigerator. The crystals formed were filtered and recrystallized from a mixture of acetone and 95% ethanol |
|
In ethanol; |
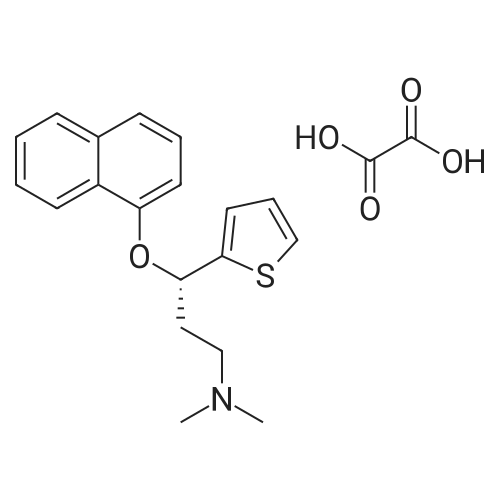
The duloxetine alkyl carbamate represented by the formula (Ib) was synthesized by a known method (steps 1 to 4 of the above scheme).Specifically, 2-acetylthiophene (63.1 g; 0.5 mol), dimethylamine hydrochloride (53.0 g; 0.65 mol), paraformaldehyde (19.8 g; 0.22 mol) and ethanol (80 ml) (1 ml) to give 3-dimethylamino-1- (2-thienyl) -1-propanone hydrochloride,Reduction with sodium borohydride, resolution with optically active mandelic acid yielded optically active (S) N, N-dimethyl-3- (2-thienyl) -3-hydroxypropylamine.Next, by reaction of 1-fluoronaphthalene with sodium hydride, N, N-dimethyl-duloxetine was obtained.Subsequently, the reaction with the corresponding alkyl chloroformate (4.6 ml; 48.5 mmol) gave the duloxetine alkyl carbamate represented by the above formula (Ib). The ethanol solution (10 mL) of the potassium hydrate (87% of purity, 2.16g;33.5mmol) was added to the toluene solution (10 mL) of the obtained duloxetine ethyl carbamate (Ib) and (2.5g;6.7mmol) at the room temperature. It heated until it flowed back at 85 to 90 degree C about mixed liquor, and the solvent was distilled off until the internal temperature became 100 degrees C from there. Repeating distilling off of a solvent furthermore, in the range of 95 to 100 degree C, temperature was maintained and it stirred for 2 hours. It cooled to the room temperature and washed the organic layer obtained by adding water (10 mL) and ethyl acetate (10 mL) with water (20 mL). With sodium sulfate, it dried, concentration drying was filtered and carried out, and the compound of the title was obtained at 1.96 g (yield: 98.4%, optical isomer:0.16%).[0054]1H-NMR(400-MHz, DMSO-d6):8.23-8.20 (m, 1H), 7.86-7.82 (m, 1H), 7.53-7.49 (m, 2H), 7.44-7.41 (m, 2H), 7.33 (t, 1H), 7.21 (dd, 1H), 7.05(d,1H),6.97(dd,1H),5.99(t,1H),2.62(2H,t)2.26(3H,s),2.36-2.29(m,1H),2.12-2.03(m,1H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping