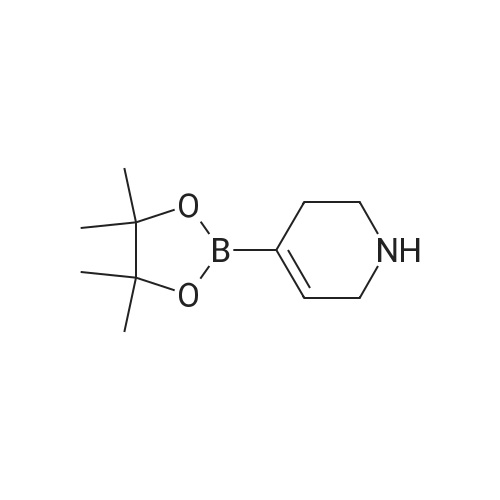
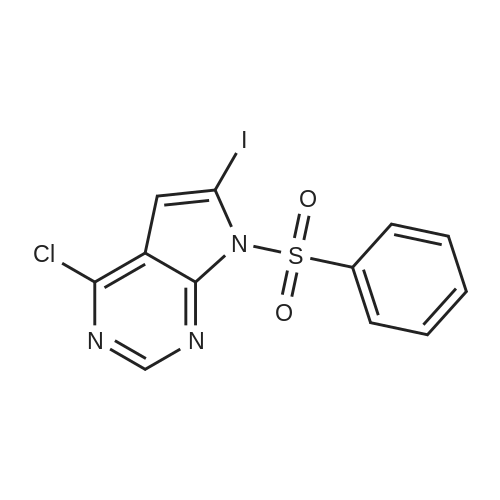
|
With potassium carbonate;bis-triphenylphosphine-palladium(II) chloride; In 1,4-dioxane; water; at 100℃;Product distribution / selectivity; |
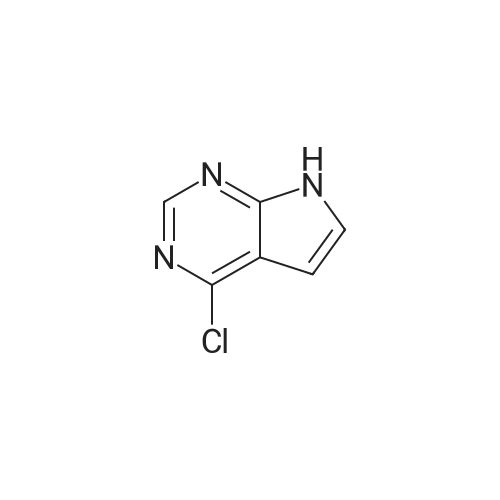
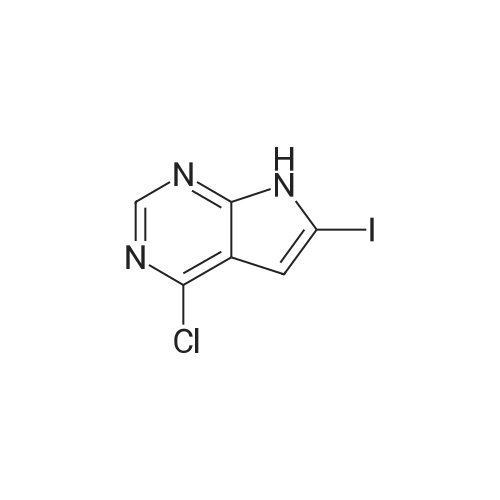
te^-ButyI4-(4-chloro-7Jy-pyrrolo[2,3-?qpyrimidm-6-yl)-3,6-dihydropyridine-l(2fl)-carboxylate (compound of Formula VIII-Boc).; IXMKCn[228] To a suspension of <strong>[876343-10-1]4-chloro-6-iodo-7H-pyrrolo[2,3-d]pyrimidine</strong> (5.0g,O.OlSmol) in 1,4-dioxane (120mL) and water (30mL) were added 4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-3,6-dihydro-2H-pyridine-l-carboxylic acid tert-butyl ester (5.97g,0.0193mol), potassium carbonate (4.9g, 0.036mol) and PdCl2(dppf)-CH2Cl2 (0.73g,0.89mmol). The flask was evacuated and refilled with N2 (3x). The mixture was heated at100C overnight. LC-MS showed the reaction was complete. The mixture was diluted withethyl acetate (200mL), then washed with brine (2x50mL), and dried over anhydrous sodiumsulfate. The filtrate was concentrated under reduced pressure to wlOOmL, the resulting whiteprecipitate was collected by filtration to give the first batch of the title compound. The filtratewas concentrated and the residue was purified by chromatography on silica gel, eluting withHex:EtOAc = 70:30 -> 50:50 to give a white solid (containing pinacol), which was furthercrystallized with EtOAc/hexanes to give the second batch of the title compound as a whitesolid. LC-MS (ES, Pos.): 335/337 (3/1) [MH+]. 'H NMR (CDC13,400 MHz): 5 = 1.51 (s,9H), 2.61 (m, 2H), 3.70 (m, 2H), 4.20 (m, 2H), 6.27 (s, 1H), 6.55 (s, 1H), 8.61 (s, 1H), 10.3(brs, 1H). |
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In propan-1-ol; water; at 100℃; for 18h; |
4-(4-(5-Fluoro-3-r4-(1-fluoro-cvclopropyl)-benzoylaminol-2-methyl-phenyl)-7H- Pyrrolor2 -dlpyrimidin-6-yl)-3,6-dihvdro-2H-pyridine-1-carboxylic acid dimethylamide(1 ) 4-(4-Chloro-7H-pyrrolor2,3-dlpyrimidin-6-yl)-3,6-dihvdro-2H-pyridine-1-carboxylic acid tert-butyl ester, Intermediate 1To a mixture of <strong>[876343-10-1]4-chloro-6-iodo-7H-pyrrolo[2,3-d]pyrimidine</strong> (2.6 g, 9.30 mmol) and dichlorobis(triphenylphosphine)palladium (II) (0.52 g, 0.74 mmol) in 1-propanol (120 ml) and aqueous sodium carbonate solution (2M, 10.23 ml, 20.46 mmol), 4-(4, 4,5,5- tetramethyl-[1 ,2,3]dioxaborolan-2-yl)-3,6-dihydro-2H-pyridine-1-carboxylic acid tert-butyl ester (3.02 g, 9.77 mmol) was added. The mixture was heated to 100C for 18 hours. After cooling the brownish mixture was diluted with 200 ml water and extracted with DCM. The organic layer was washed with brine (2x) and dried over sodium sulfate, than filtered and evaporated. The residue was purified by flash chromatography on silica (cyclohexane/EtOAc 1 :1 ) to afford the compound Intermediate 1 as a beige solid.MS (ESI): 335 [M+H]+ , 1H-NMR (DMSO-d6): delta (ppm) 12.64 (br s, 1 H), 8.56 (s, 1 H), 6.59 (s, 2H), 4.08 (br s, 2H), 3.57 (m, 2H), 2.55 (m, 2H), 1.45 (s, 9H). (2) 4-Chloro-6-(1.2.3.6-tetrahvdro-pyridin-4-vn-7H-pyrrolor2.3-dlpyrimidine. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping