|
With methanol; sodium tetrahydroborate; at 0 - 20℃; for 6h; |
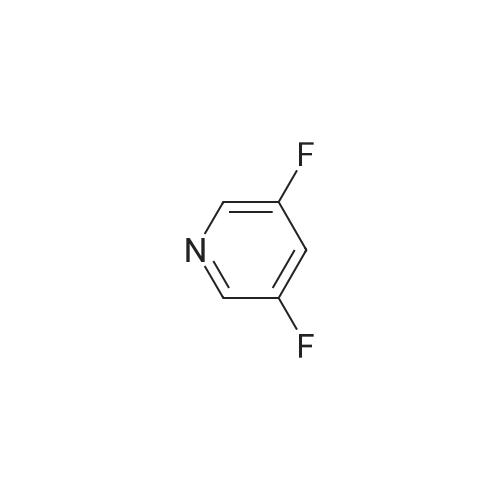
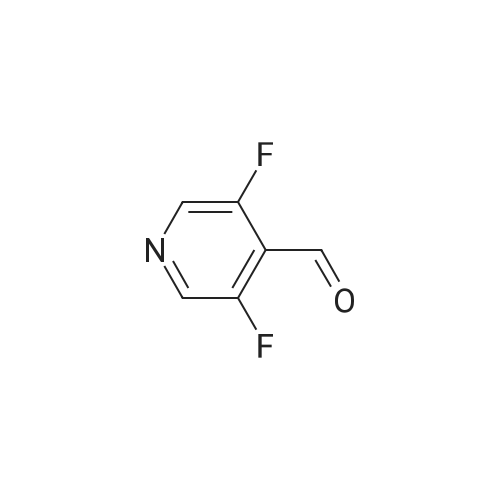
To a solution of sodium borohydride (198 mg, 5.2 mmol) in anhydrous methanol (8 mL) was added a solution of <strong>[870234-98-3]3,5-difluoropyridine-4-carbaldehyde</strong> (0.5 g, 3.5 mmol) in anhydrous methanol (2 mL) at O0C. The mixture was stirred for 6 hours at room temperature. Water was added, and the mixture was extracted 3 times with ethyl acetate. The organic phase was washed with brine and dried over sodium sulfate.After evaporation of the solvent, (3,5-difluoropyridin-4-yl)methanol was obtained and used in the next step without purification; 1H NMR (400 MHz, CDCl3): δ 2.25 (bs, IH), 4.85 (s, 2H), 8.36 (s, 2H). |
| 203 mg |
With methanol; sodium tetrahydroborate; at 0 - 20℃; for 5h; |
Step 2: (3,5-difluoro-4-pyridyl)methanolNaBH4 (0.0788 g, 2.08 mmol) was dissolved in 3.2ml_ MeOH (H2-formation). At 0C a solution of <strong>[870234-98-3]3,5-difluoropyridine-4-carbaldehyde</strong> (0.200 g, 1 .40 mmol) in 0.8ml_ MeOH was added dropwise (H2-formation). The reaction mixture was stirred 5h at rt. Water was added dropwise and the mixture was extracted 3 times with EtOAc. The combined organic phases were washed with brine. The organic phase was dried with Na2SC>4 , filtrated and the filtrate evaporated. The desired product was obtained as a white solid (203 mg), 1H-NMR (CDCIs): 8.37 (s, 2H), 4.83 (s, 2H), 2.20 (bs, 1 H). |
|
With sodium tetrahydroborate; |
Example 26A (3,5-Difluoropyridin-4-yl)methanol At RT, 11.60 g of <strong>[870234-98-3]3,5-difluoroisonicotinaldehyde</strong> (Example 25A, 81 mmol, 1 equivalent), dissolved in 100 ml of methanol, were added to 3.68 g of sodium borohydride (97.3 mmol, 1.2 equivalents) in 200 ml of methanol. After the evolution of gas had ended (about 2 h), 200 ml of saturated aqueous sodium chloride solution were added and the mixture was extracted twice with in each case 200 ml of ethyl acetate. The combined organic phases were dried over sodium sulphate and concentrated. This gave 9.5 g (81% of theory) of the title compound. LC-MS (Method 2): Rt=0.28 min MS (ESpos): m/z=146 (M+H)+ 1H-NMR (400 MHz, DMSO-d6): δ=4.56 (d, 2H), 5.56 (t, 1H), 8.51 (s, 2H). |
|
With sodium tetrahydroborate; In methanol; at 0℃; for 1h; |
A solution of 3, 5-difluoropyridine-4-carbaldehyde (500 mg, 3.49 mmol, 1 .00 equiv) in methanol (10 mL) was cooled to 0 C, treated with aBH4 (133 mg, 3.52 mmol, 1.01 equiv), and stirred for 1 h at 0 C. The reaction was then quenched by the additio of 10 mL of water, concentrated under vacuum, extracted with 2x15 mL of ethyl acetate and the organic layers combined and concentrated under vacuum. The residue was applied onto a. silica gel column with ethyl acetate/petroleum ether (1 :3) to give the title compound as a yellow solid. Mass spectrum (ESI, m/z): Calcd. for C6H5F2NO, 146.0 (M+H), found 146.0. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping