| 94% |
With water; sodium hydroxide; In methanol; at 0 - 20℃; for 15h; |
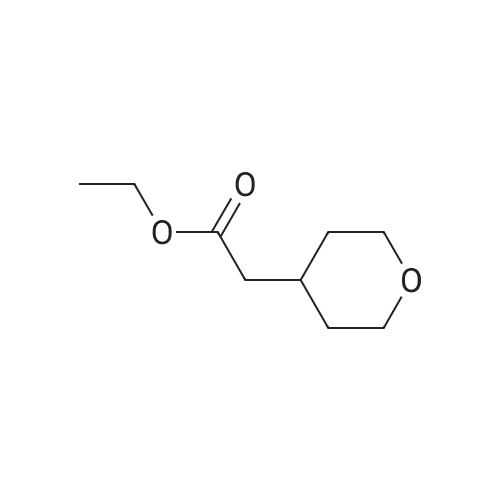
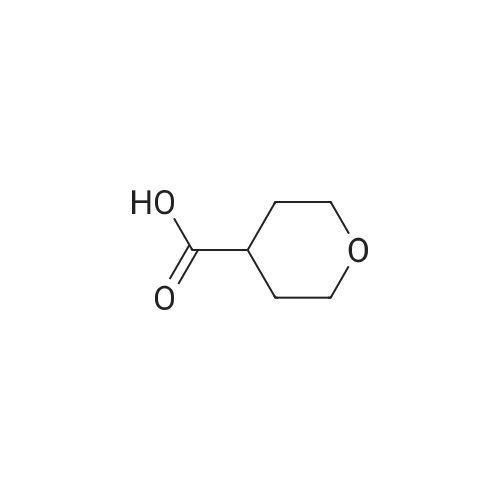
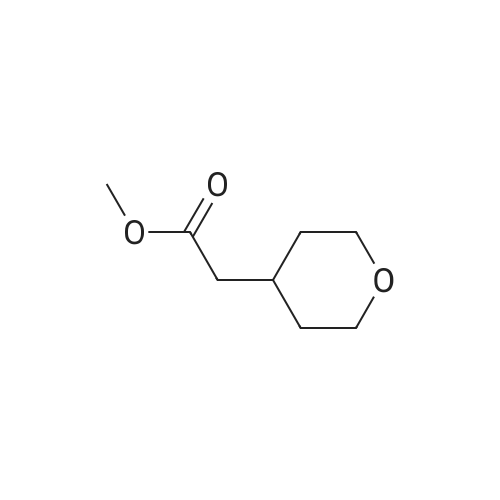
A solution of sodium hydroxide (15.9 g, 44.06 mmol) in water (150 ml) was added dropwise to a solution of <strong>[103260-44-2]ethyl 2-(tetrahydro-2H-pyran-4-yl)acetate</strong> (15 g, 8.81 mmol) in methanol (150 ml) at a temperature of 0 C or below, and the mixture was stirred at room temperature for 15 hr. The solvent of the reaction mixture was removed by distillation under reduced pressure, and the water layer was adjusted to pH 3 by the addition of 1 M hydrochloric acid and was extracted with ethyl acetate. The organic layer was washed with saturated brine and was dried over anhydrous sodium sulfate, and the filtrate was concentrated under reduced pressure to give a corresponding acid as a colorless solid (12.0 g, 94%). This compound was used in the next step without further purification. Benzyl bromide (14.3 g, 83.33 mmol) was added dropwise to a suspension of this compound (10.0 g, 69.44 mmol) and anhydrous potassium carbonate (28.8 g, 208.3 mmol) in acetonitrile (100 ml) at room temperature, and the mixture was refluxed for 48 hr. The solvent of the mixture was removed by distillation under reduced pressure, the residue was diluted with water and was extracted with dichloromethane, and the organic layer was dried over anhydrous sodium sulfate. The filtrate was concentrated under reduced pressure, and the residue was chromatographed on silica gel column (hexane:ethyl acetate = 9:1) to give the tile compound as an oil (12.0 g, yield 75%). 1H-NMR (400 MHz, CDCl3): delta (ppm) 7.39-7.34 (m, 5H), 5.12 (s, 2H), 3.95-3.92 (m, 2H), 3.42-3.36 (m, 2H), 2.30 (d, J = 7.2 Hz, 2H), 2.09-1.99 (m, 1H) 1.64-1.61 (m, 2H), 1.39-1.29 (m, 2H); MS (ESI): m/z 234 (M+). |
|
With sodium hydroxide; In methanol; water; |
Preparatory Example 33 4-Tetrahydropyranylacetic acid STR234 20 ml of methanol, 10 ml of water and 1 g of sodium hydroxide were added to 0.9 g of ethyl 4-tetrahydropyranylacetate and agitated at 80 C. for 1 hour. The solvent was removed by distillation, to which water was added. After washing with ethyl acetate, a hydrochloric acid aqueous solution was added to the resultant aqueous phase to an extent of pH of 3, followed by extraction with chloroform under salting-out conditions and drying with anhydrous magnesium sulfate. This was removed by filtration and the solvent was distilled off, thereby obtaining 0.87 g of a crude intended compound. 1 H-NMR(CDCl3) delta:1.0-2.4(m,5H), 2.28(bd,J=6.5 Hz,2H), 3.37(td,J=11.5 Hz,2.9 Hz,2H), 3.7-4.1(m,2H), 7.85(bs,1H) |
|
With potassium hydroxide; In ethanol; for 18h; |
The product of Preparative Example 11 (3.04) g, 17.7 mmol) was dissoloved in 90 mL of ethanol containing 3 g (53 mmol) of potassium hydroxide. This was stirred for 18 hours and then concentrated under vacuum. The residue was dissolved in 15 mL of water, adjusted to pH 2 with 12 N HCl, and extracted with three 50 mL portions of dichloromethane. The combined organic layers were dried over magnesium sulfate and concentrated under vacuum giving 2.04 g of the product as a white solid, mp = 60-63C. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +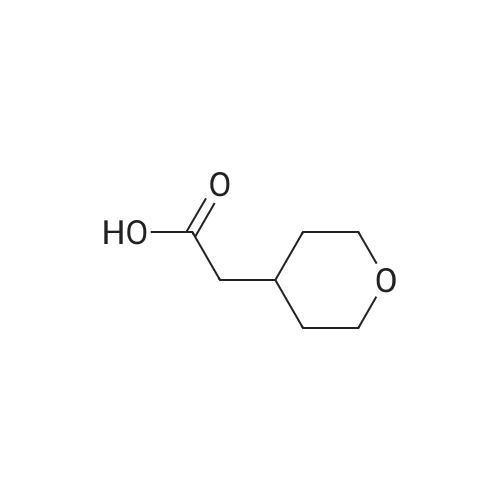

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping