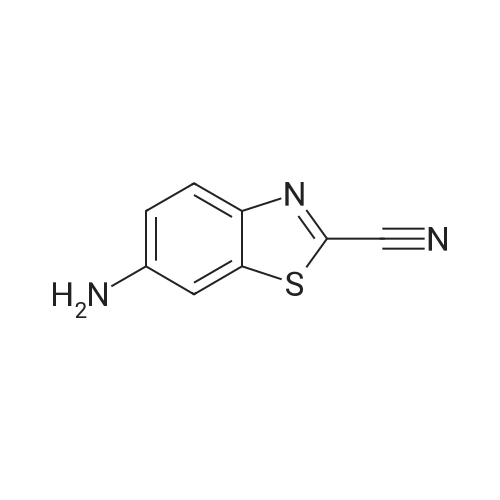
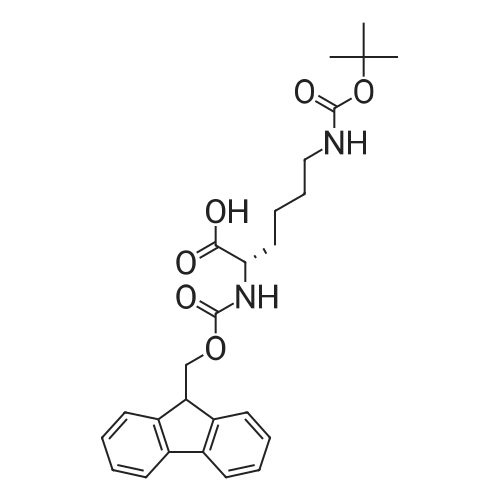
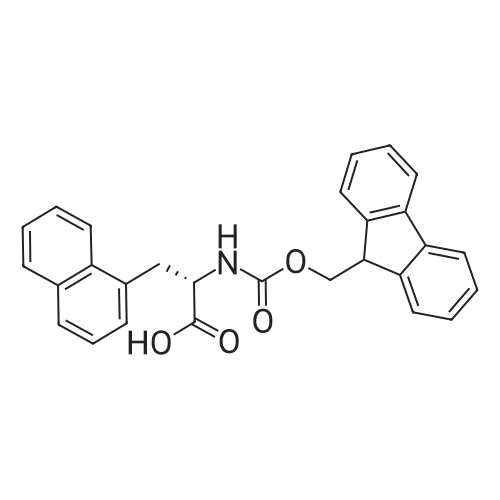
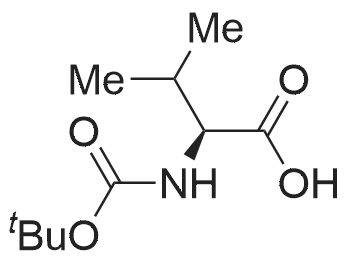
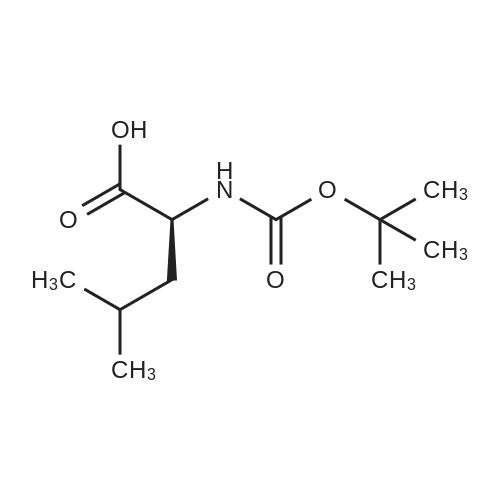
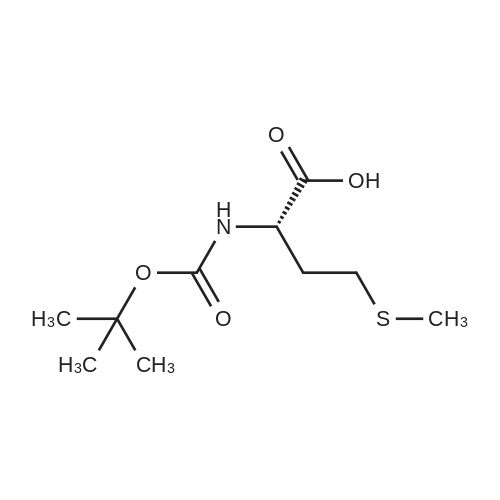
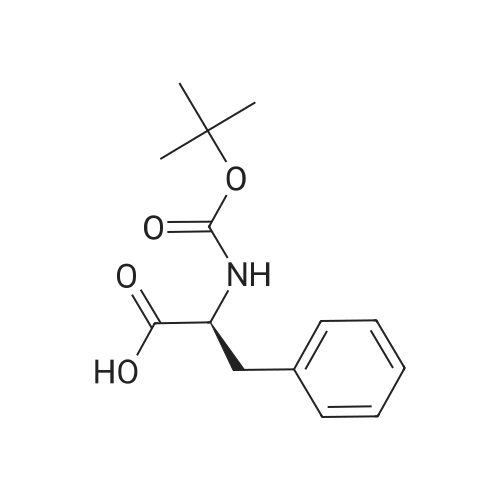
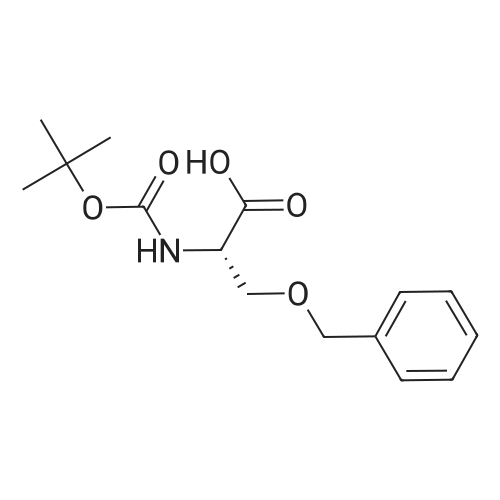
|
|
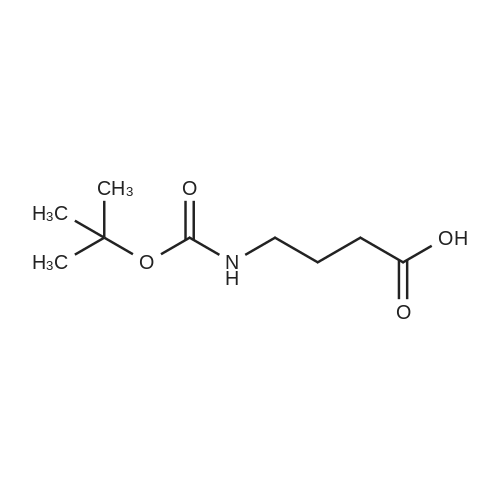
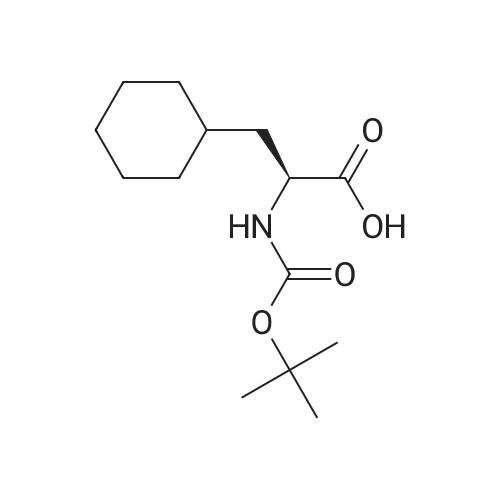
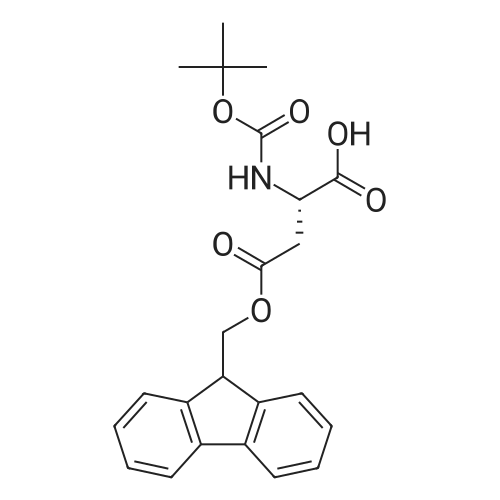
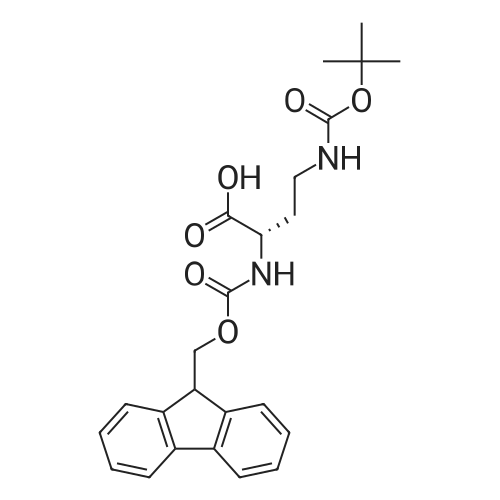
The title peptide was synthesized on an Applied Biosystems (Foster City, Calif.) model 430A peptide synthesizer which was modified to do accelerated Boc-chemistry solid phase peptide synthesis. See Schnolzer, et al., Int. J. Peptide Protein Res., 40:180 (1992). 4-methylbenzhydrylamine (MBHA) resin (Peninsula, Belmont, Calif.) with the substitution of 0.91 mmol/g was used. The Boc amino acids (Novabiochem, San Diego, Calif. and Chem-Impex, Wood Dale, Ill.) used were: Boc-Cha-OH, Boc-Asp(OFm)-OH, Boc-His(DNP)-OH, Boc-D-Phe-OH, Boc-Arg(Tos)-OH, <strong>[47355-10-2]<strong>[47355-10-2]Boc-Trp(For)</strong>-OH</strong>, Boc-Gaba-OH, and Boc-Lys(Fmoc)-OH. The synthesis was carried out on a 0.20 mmol scale. The Boc groups were removed by treatment with 100% TFA for 2×1 minute. Boc amino acids (2.5 mmol) were pre-activated with HBTU (2.0 mmol) and DIEA (1.0 mL) in 4 mL of DMF and were coupled without prior neutralization of the peptide-resin TFA salt. Coupling times were 5 minutes. (0223) At the end of the assembly of Boc-Asp(OFm)-His(DNP)-D-Phe-Arg(Tos)-Trp(For)-Gaba-Lys(Fmoc)-MBHA, the peptide-resin was transferred into a reaction vessel on a shaker. The resin was treated twice with 25% piperidine in DMF for 15 minutes per session, washed with DMF, and shaken with bromo-tris-pyrrolidino-phosphonium hexafluorophosphate (PyBrOP) (6 eq, 0.3 mmol), DIEA (1 mL), and 4-(dimethylamino)pyridine (DMAP) (24 mg) in DMF (2 mL) for 12 hours. After washing with DMF, the resin was treated twice with 100% TFA for 2 minutes per treatment, washed with DMF and DCM, and then dried under reduced pressure. One fourth of the peptide-resin (0.05 mmol) was used for the next coupling with Boc-Cha-OH (10 eq, 0.5 mmol) in the presence of HBTU (9 eq, 0.45 mmol) and DIEA (0.25 mL) in DMF for 10 minutes. After the deprotection with 100% TFA in two sessions lasting approximately 2 minutes each, the peptide-resin was then washed with DMF. The final capping step was done by shaking the resin with acetic anhydride (40 eq, 2.0 mmol) and DIEA (20 eq, 1.0 mmol) in DMF for 1 hour. After washing with DMF, the resin was treated twice with a solution of 20% mercaptoethanol/10% DIEA in DMF, each treatment lasting approximately 30 minutes, to remove the DNP group on the Histidine side chain. The formyl group on the side chain of Tryptophan was removed by shaking with a solution of 15% ethanolamine/15% water/70% DMF twice for 30 minutes per shaking. The peptide-resin was washed with DMF and DCM and dried under reduced pressure. The final cleavage was done by stirring the peptide-resin in 10 mL of HF containing 1 mL of anisole and dithiothreitol (30 mg) at 0 C. for 75 minutes. HF was removed by a flow of nitrogen. The residue was washed with ether (6×10 mL) and extracted with 4N HOAc (6×10 mL). (0224) The peptide mixture in the aqueous extract was purified on reverse-phase preparative high pressure liquid chromatography (HPLC) using a reverse phase VYDAC C18 column (Nest Group, Southborough, Mass.). The column was eluted with a linear gradient (10% to 50% of solution B over 40 minutes) at a flow rate of 10 mL/minute (Solution A=water containing 0.1% TFA; Solution B=acetonitrile containing 0.1% of TFA). Fractions were collected and checked on analytical HPLC. Those containing pure product were combined and lyophilized to dryness. 5.1 mg of a white solid was obtained. Yield was 8.9%. Purity was 94.5% based on analytical HPLC analysis. Electro-spray mass spectrometer (MS(ES))S analysis gave the molecular weight at 1148.5 (in agreement with the calculated molecular weight of 1148.3). (0225) Other peptides of the invention can be prepared by a person of ordinary skill in the art using synthetic procedures analogous to those disclosed generally hereinabove and/or to those disclosed specifically in the foregoing examples, as were the compounds depicted in Tables 1A and 1B. (0226) Other peptides of the invention can be prepared by a person of ordinary skill in the art using synthetic procedures analogous to those disclosed generally hereinabove and/or to those disclosed specifically in the foregoing examples, as were the compounds depicted in Tables 1A and 1B. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping