|
With thionyl chloride; In toluene; at 110℃; for 1.5h; |
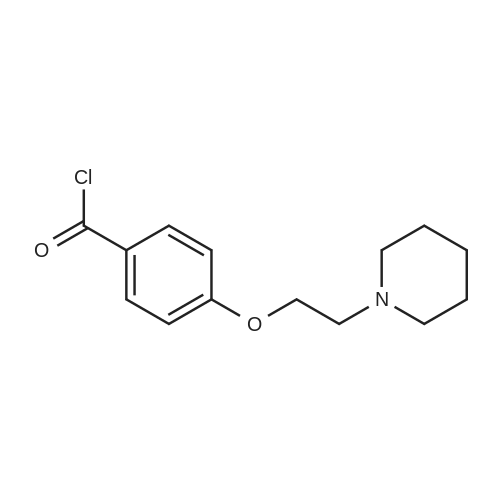
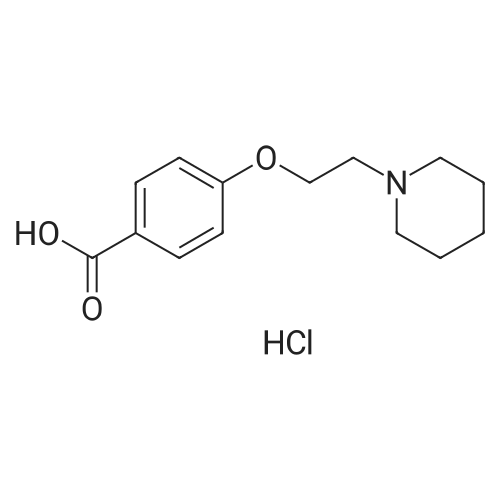
To a solution of <strong>[84449-80-9]4-(2-piperidin-1-ylethoxy)benzoic acid hydrochloride</strong> (7.0 g) in thionyl chloride (70 ml) was added toluene (70 ml), the solution was stirred for 1.5 hours at 110°C, then the solvent was evaporated in vacuo. To a suspension of the resulting 4-(2-piperidin-1-ylethoxy)benzoyl chloride hydrochloride (4.5 g) in dichloromethane (100 ml) were sequentially added 3-bromoanisole (1.7 ml) and aluminum chloride (4.1 g) on an ice bath under a nitrogen atmosphere, and the solution was stirred overnight at room temperature. Tetrahydrofuran and aqueous ammonia were sequentially added thereto on the ice bath, the solution was filtered through celite pad, anhydrous magnesium sulfate was added thereto followed by stirring. The residue obtained by filtration, and then evaporation of the solvent in vacuo, was purified by NH silica gel column chromatography (hexane-ethyl acetate system) to provide the title compound (2.8 g).1H-NMR (400MHz, CDCl3); delta (ppm): 1.41-1.50 (m, 2H), 1.57-1.64 (m, 4H), 2.46-2.54 (m, 4H), 2.79 (t, 2H), 3.86 (s, 3H), 4.17 (t, 2H), 6.92 (dd, 1H), 6.93 (d, 2H), 7.17 (d, 1H), 7.29 (d, 1H), 7.77 (d, 2H). |
|
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 20℃; for 2h; |
To a solution of <strong>[84449-80-9]4-(2-piperidin-1-ylethoxy)benzoic acid hydrochloride</strong> (1.0 g) in dichloromethane solution (20 ml) were sequentially added oxalyl chloride (0.4 ml) and N,N-dimethylformamide (0.05 ml), the solution was stirred for 2 hours at room temperature, and then the solvent was evaporated in vacuo. To the residue were sequentially added tetrahydrofuran (20 ml), N,N-diisopropylethylamine (5 ml) and 6-(2-amino-4-methoxyphenyl)-2-methoxy-5,6,7,8-tetrahydronaphthalene (800 mg), and the solution was stirred for 30 minutes at room temperature. To the reaction solution was added a saturated aqueous solution of sodium bicarbonate, the solution was extracted with ethyl acetate, then washed with brine, dried over anhydrous magnesium sulfate, and then the solvent was evaporated in vacuo. Obtained by purifying the residue by NH silica gel column chromatography (hexane-ethyl acetate system), to a solution of the resultingN-[5-methoxy-2-(6-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)phenyl]-4-(2-piperidin-1-ylethoxy)benzamide (1.1 g) in tetrahydrofuran (30 ml) was added lithium aluminum hydride (0.4 g), and the solution was refluxed for 20 minutes. The solution was cooled on an ice, then ammonia solution (1 ml) and anhydrous magnesium sulfate was added, the solution was filtered, then the solvent was evaporated in vacuo. To a solution of the total amount of the resulting [5-methoxy-2-(6-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)phenyl] [4-(2-piperidin-1-ylethoxy)benzyl]amine (crude product) in dichloromethane (15 ml) were added pyridine (0.5 ml) and acetic anhydride (0.4 ml), and the solution was stirred for 1 hour at room temperature. To the reaction solution was added a saturated aqueous solution of sodium bicarbonate, the solution was extracted with ethyl acetate, then washed with brine, dried over anhydrous magnesium sulfate, then the solvent was evaporated in vacuo. To a solution of the resultingN-[5-methoxy-2-(6-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)phenyl]-N-[4-(2-piperidin-1-ylethoxy)benzyl]acetamide (1.3 g) in tetrahydrofuran (30 ml) was added lithium aluminum hydride (0.4 g), and the solution was refluxed for 20 minutes. The solution was cooled on an ice, then ammonia solution (1 ml) and anhydrous magnesium sulfate were added thereto, the solution was filtered, and then the solvent was evaporated in vacuo. Obtained by purifying the residue by NH silica gel column chromatography (hexane-ethyl acetate system), ethyl [5-methoxy-2-(6-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)phenyl] [4-(2-piperidin-1-ylethoxy)benzyl]amine (570 mg) was used according to an analogous synthetic method to Example 111 to provide the title compound (480 mg).1H-NMR (400MHz, CDCl3); delta (ppm): 0.93 (t, 3H), 1.44-1.47 (m, 2H), 1.62-1.75 (m, 6H), 2.21-2.63 (m, 6H), 2.77-2.80 (m, 4H), 2.86-2.92 (m, 2H), 3.56-3.59 (m, 1H), 3.89 (s, 2H), 4.07 (t, 2H), 6.57-6.61 (m, 3H), 6.66 (d, 1H), 6.69 (d, 2H), 6.86 (d, 2H), 7.05 (d, 2H), 7.08 (d, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping