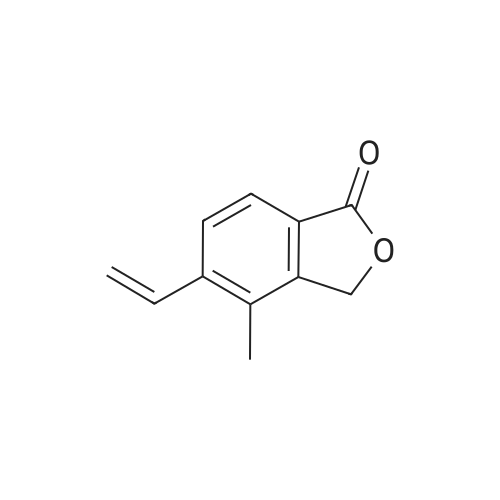
| 2 g |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium hydrogencarbonate; In ethanol; toluene; at 80℃; for 0.5h;Inert atmosphere; |
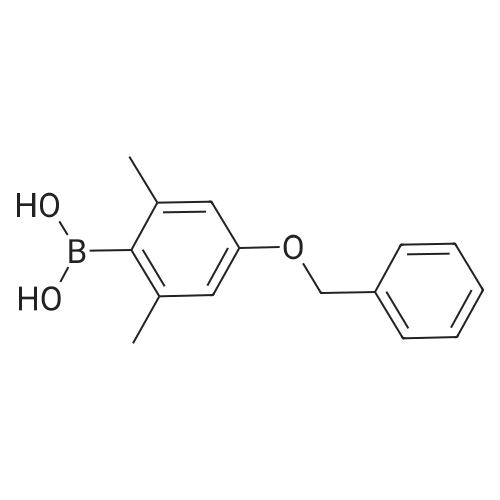
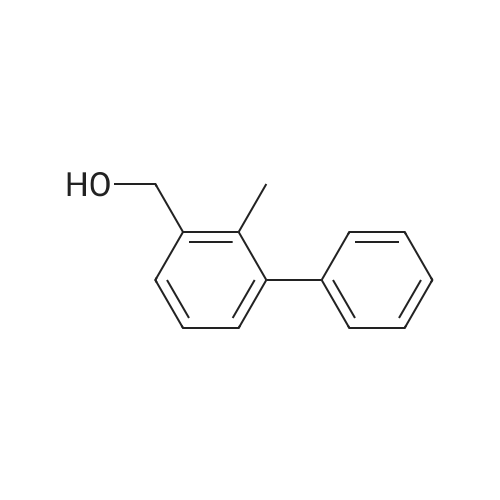
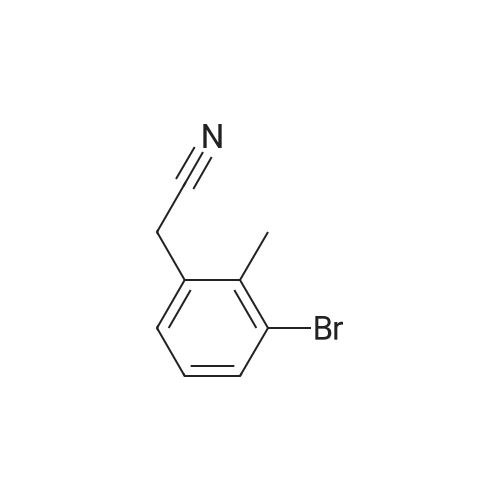
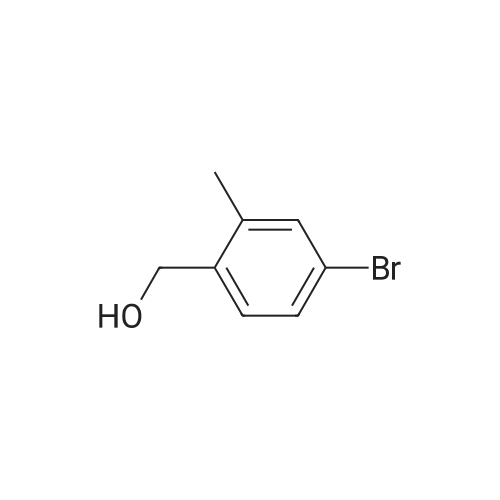
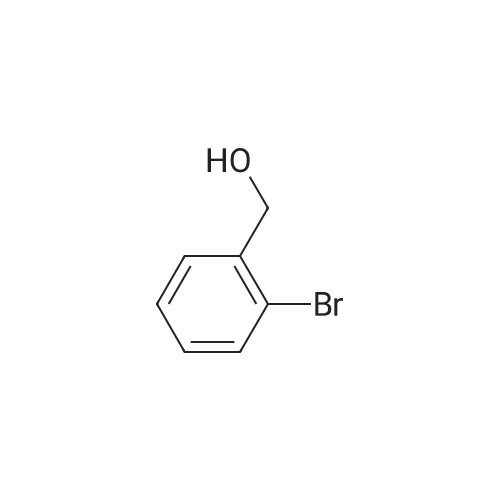
A mixture of (3-bromo-2-methylphenyl)methanol (2.071 g, 10.3 mmol), phenylboronic acid (2.51 g, 20.60 mmol) and [l,l'-bis(diphenylphosphino)ferrocene] dichloropalladium (II) dichloromethane complex (0.084 g, 0.103 mmol) in toluene (15.45 ml) and ethanol (5.15 ml) was placed under argon. To this solution was added sodium bicarbonate, 2M (15.45 ml, 30.9 mmol) and the mixture was heated at 80 C for 30 min. The reaction mixture was diluted with 20 mL ethyl acetate and 5 mL water. The organic portion was concentrated by rotatory evaporation. The crude product was chromatographed on silica gel eluting with 0-40% ethyl acetate in hexane to afford 2 g of an off-white solid. 1H NMR (400MHz, CHLOROFORM-d) delta 7.47-7.29 (m, 7H), 7.23 (s, 1H), 4.80 (d, J=5.6 Hz, 2H), 2.27 (s, 3H), 1.63-1.59 (m, 1H). |
| 2 g |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium hydrogencarbonate; In ethanol; toluene; at 80℃; for 0.5h;Inert atmosphere; |
A mixture of (3-bromo-2-methylphenyl)methanol (2.071 g, 10.3 mmol), phenylboronic acid (2.51 g, 20.60 mmol) and [1,1?-bis(diphenylphosphino)ferrocene]dichloropalladium (II) dichloromethane complex (0.084 g, 0.103 mmol) in toluene (15.45 mL) and ethanol (5.15 mL) was placed under argon. To this solution was added sodium bicarbonate, 2M (15.45 mL, 30.9 mmol) and the mixture was heated at 80 C. for 30 minutes. The reaction mixture was diluted with 20 mL ethyl acetate and 5 mL water. The organic portion was concentrated by rotatory evaporation. The crude product was chromatographed on silica gel eluting with 0-40% ethyl acetate in hexane to afford 2 g of an off-white solid. 1H NMR (400 MHz, CHLOROFORM-d) delta 7.47-7.29 (m, 7H), 7.23 (s, 1H), 4.80 (d, J=5.6 Hz, 2H), 2.27 (s, 3H), 1.63-1.59 (m, 1H). |
| 4.58 g |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium hydrogencarbonate; In ethanol; toluene; at 80℃; for 0.5h;Inert atmosphere; |
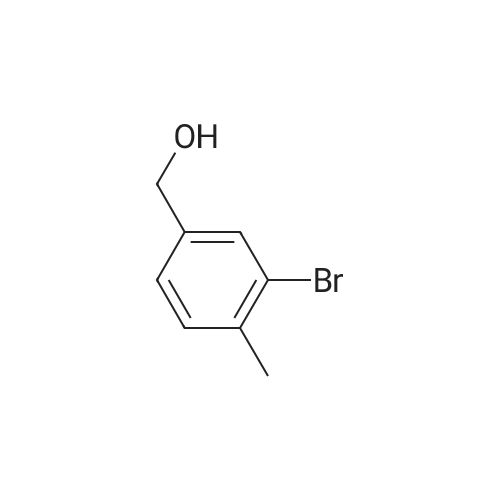
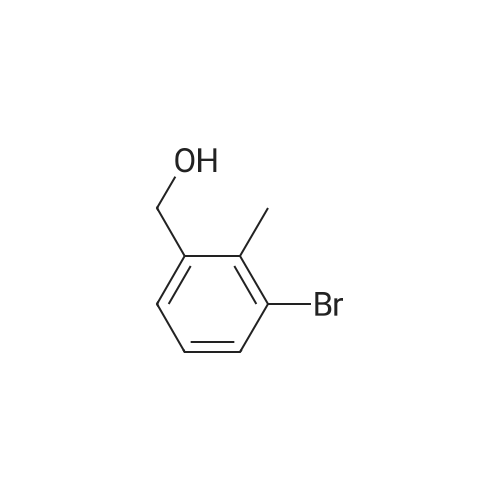
A mixture of compound 2 (4.6 g, 22.8 mmol), phenylboronic acid 3 (5.65 g, 46.3 mmol) and [ 1 , 1 ' -bis (diphenylphosphino) - ferrocene ] dichloropalladium ( I I ) dichloromethane complex (0.188 g, 0.103 mmol) in toluene (34.5 mL) and ethanol (11.3 mL) was placed under argon. To this solution sodium bicarbonate, 2M (34.5 mL, 69.0 mmol) was added and the mixture was heated at 80 C for 30 min. Ethyl acetate (44 mL) and (11 mL) water were added to the reaction mixture. The organic extract was concentrated by rotatory evaporation. The crude product was chromatographed on silica gel eluting with 0-40% ethyl acetate in hexane to afford 4.58 g of an off-white solid, mp: 58.0- 59.5 C; 1H NMR (600 MHz, CDC13) delta [ppm] : 7.43-7.40 (m, 3H) , 7.35 (m, 1H) , 7.31-7.29 (m, 2H) , 1H) , 7.26 (t, J=7.6 Hz, 1H) , 7.20 (dd, Jl=7.6 Hz, J2=1.3 Hz, 1H) , 4.78 (s, 2H) , 2.25 (s, 3H) ; 13C NMR (151MHz, DMSO-d6) delta [ppm]: 143.0, 142.2, 140.0, 133.8, 129.7, 129.5, 128.2, 127.0, 126.9, 125.7, 64.2, 16.0; IR V (ATR cm-1) : 3365, 3054, 1601, 1469, 1047, 757. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping