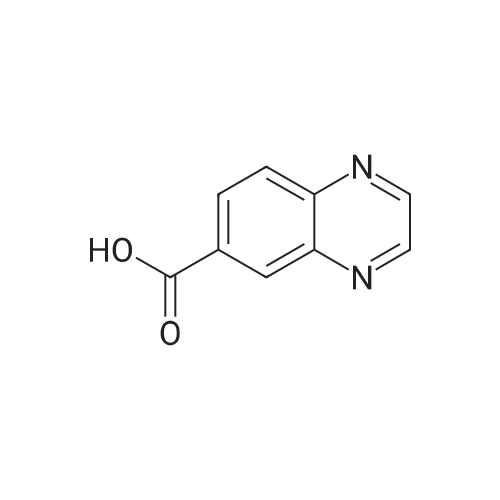
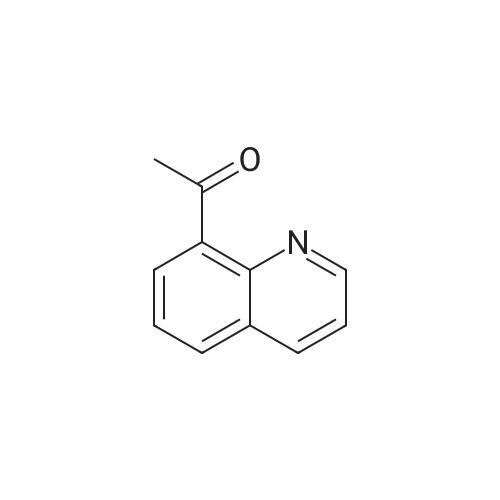
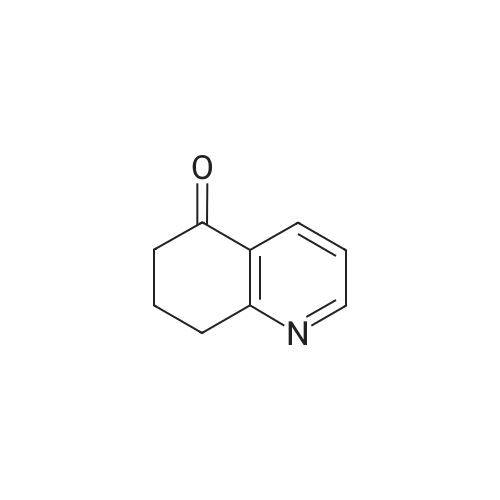
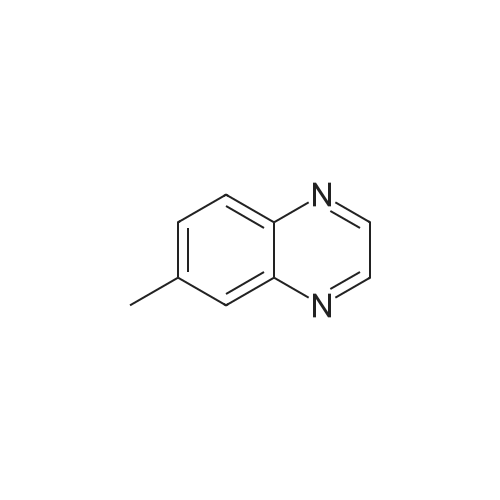
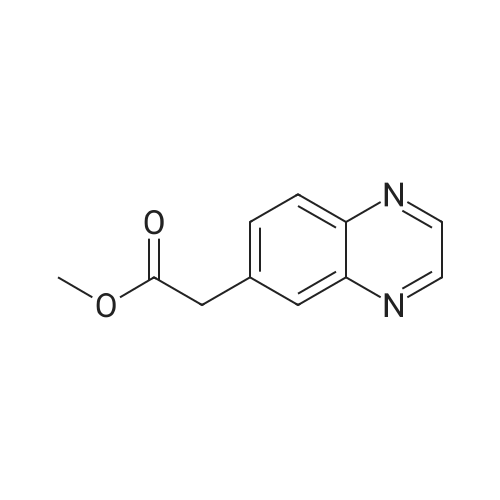
| 75.7% |
at 0 - 20℃; for 2 h; Inert atmosphere |
To a solution of Compound 2 (1 g, 4.6 mmol) in dry THF (10 mL) at 0 °C under N2 was added MeMgBr (2 mL, 3.0 mol/L, 6 mmol) dropwise. The resulting solution was slowly warm to RT over 2 hours. The mixture was diluted with NH4C1 solution and extracted with EA. The organic extracts were concentrated to give a crude oil. The crude product was purified by silica gel chromatography to afford Compound 3 (600 mg, 75.7 percent). |
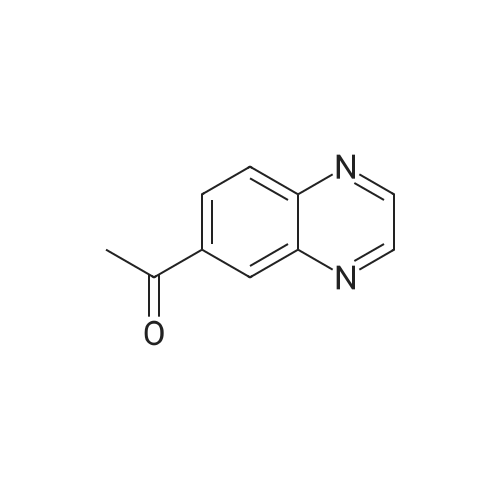
| 74% |
at 0 - 20℃; for 3 h; |
A mixture of quinoxaline-6-carboxylic acid (2 g, 11.49 mmol) and thionyl chloride (30 mL) was stirred at reflux for 2 hours. The reaction mixture was concentrated to dryness using a rotary evaporator to afford quinoxaline-6- EPO <DP n="101"/>carboxylic acid chloride (crude quantitative). A solution of the above acid chloride (11.49 mmol) in DCM (50 mL) and pyridine (20 mL) was mixed with N,O-dimethyl hydroxylamine HCI salt (2.24 g, 23 mmol) and stirred at room temperature for 12 hours. The reaction was quenched by adding aqueous HCI (50 mL, 1 N), extracted with DCM (3x100 mL), concentrated using a rotary evaporator. The residue was further purified by column (Siψ2, Hexanes/EtOAc = 1 :3) to yield quinoxaline-6-carboxylic acid methoxy-methyl-amide (2 g, 80percent). To a solution of the above Weinreb amide (2.0 g, 9.2 mmol) in THF (30 mL) at O0C was added methyl magnesium bromide (3.9 mL, 11.6 mmol). The reaction mixture was stirred at O0C for 2 hours and then 1 hour at room temperature, quenched by adding aqueous HCI (20 mL, 1 N), extracted with DCM (3x100 mL), concentrated using a rotary evaporator. The residue was further-purified by column (SiO2, Hexanes/EtOAc = 1 :3) to yield 6-acetylquinoxaline (1.17 g, 74percent). A solution of 2- chloronicotinic acid ethyl ester (5.0 g, 27 mmol) in MeOH (25 mL) was mixed with sodium methoxide (25.6 mL, 112.5 mmol) and stirred at reflux for 12 hours. The reaction was quenched by adding water (100 mL), extracted with DCM (3x100 mL), concentrated using a rotary evaporator to afford 2-methoxynicotinic acid methyl ester (3.2 g, 71percent). A solution of 6-acetylquinoxaline (0.62 g, 3.6 mmol), 2- methoxynicotinic acid methyl ester (0.64 g, 3.8 mmol), and sodium hydride (0.46 g, 11.4 mmol) in THF (100 mL) was stirred at room temperature for 16 hours. The reaction was quenched by adding water (100 mL) and AcOH (20 mL), extracted with dichloromethane (3x100 mL), and concentrated using a rotary evaporator. The residue was re-dissolved in DCM (5 mL) and MeOH (3 mL) and was diluted with Hexanes (50 mL). The solid was removed by filtration and the filtrate was concentrated to afford the diketo compound (0.7 g, 60percent). A solution of the above diketone (0.4 g, 1.3 mmol) in AcOH (50 mL) and sulfuric acid (cone, 15 drops) was stirred at reflux for 1 hour. Most of the solvent was removed using a rotary evaporator. The residue was re-dissolved in MeOH and neutralized with potassium carbonate to pH = 8. The solid residue was removed by filtration, washed with MeOH and DCM. The filtrate was extracted with CH2CI2 (3x100 mL) and concentrated using a rotary evaporator. The solid residue was purified by column (SiO2, Hexanes/EtOAc/MeOH = 2:2:1) to afford 2-(quinoxalin-6-yl)-4H- EPO <DP n="102"/>pyrano[2,3-b]pyridin-4-one (90 mg, 24percent); MS (ES) m/z: 276 (M+1 ); MP 272.3- 274.80C |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping