|
With 2,6-dimethylpyridine; 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In N,N-dimethyl-formamide; |
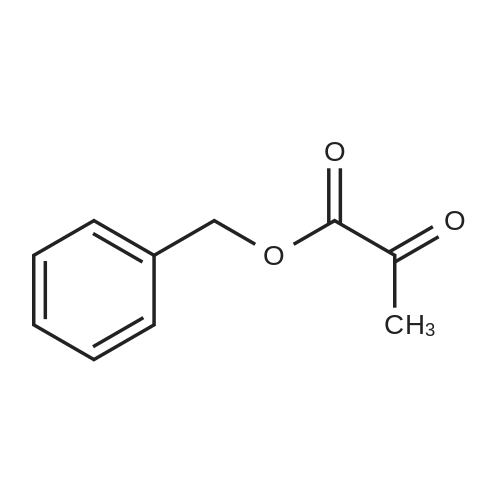
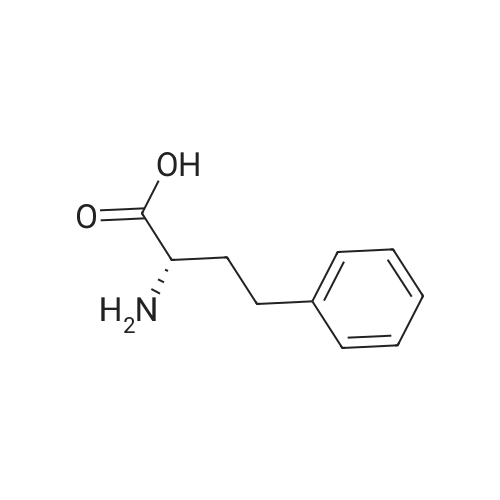
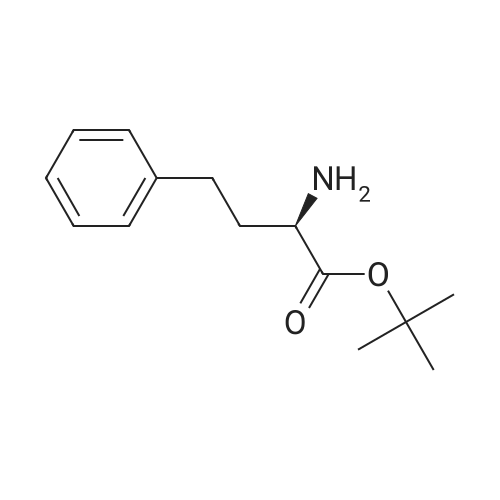
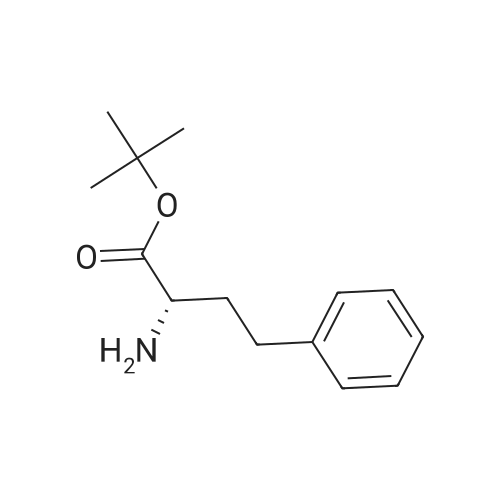
General Procedure for Amine Coupling with Acid Carboxylic acid (1.00 mmol) , amine (1.00 mmol, 1 equiv) , and l-hydroxy-7-aza-benzotriazole (HOAt) (1.10 mmol, 1.1 equiv) were combined in a 20 mL scintillation vial equipped with a stir bar. Anhydrous DMF (5 mL) and 2,6-lutidine (5.0 mmol, 5.0 equiv) were added, and the mixture stirred until complete dissolution of the reagents. l-Ethyl-3- ( 3- dimethylaminopropyl ) carbodiimide (EDCI · HC1 ) (1.05 mmol, 1.05 equiv) was added, and the mixture was stirred 12-24 hours. The reaction mixture was diluted with EtOAc (30 mL) , washed with 0.1 N HC1 (2 χ 25 mL) and sat. aqueous NaCl (25 mL) . The aqueous phase was extracted with EtOAc (2 χ 10 mL) , and combined organic phases were dried over Na2SC>4, decanted and concentrated. Purified products were isolated by flash chromatography. ( M-1-458) The general procedure for amine coupling with acid was followed: Carboxylic acid M- 1-52/457 (44 mg, 0.165 mmol) , <strong>[83079-77-0]HoPhe-OtBu</strong> (39 mg, 0.165 mmol, 1 equiv) , HOAt (25 mg, 0.181 mmol, 1.1 equiv) , 2,6-lutidine (95 μι , 0.823 mmol, 5.0 equiv) and EDCI'HCl (33 mg, 0.173 mmol, 1.05 equiv) were employed. Flash chromatography (S1O2, 10% EtOAc/hexanes ) afforded 11.4 mg (14%) of coupled product. XH NMR (400 MHz, CDC13) δ 8.71 - 8.66 (m, 1H) , 7.77 - 7.72 (m, 2H) , 7.58 - 7.47 (m, 2H) , 7.47 - 7.40 (m, 2H), 7.34 - 7.28 (m, 3H) , 7.21 (d, J = 7.3 Hz, 3H) , 6.71 (d, J = 7.7 Hz, 1H) , 4.81 ( ddd, J = 7.6, 6.6, 5.1 Hz, 1H) , 2.84 - 2.62 (m, 2H) , 2.32 (dddd, J = 13.8, 10.3, 6.5, 5.1 Hz, 1H) , 2.13 (dddd, J = 13.8, 12.3, 10.9, 6.0 Hz, 1H) , 1.53 (s, 9H) . (M -1-87) The general procedure for amine coupling with acid was followed: alkene carboxylic acid MM-1-86 (300 mg, 0.66 mmol, 1.00 equiv), HoPhe- OtBu (155 mg, 0.66 mmol, 1.00 equiv), HOAt (99 mg, 0.72 mmol, 1.10 equiv), 2 , 6-lutidine (0.385 mL, 3.29 mmol, 5.00 equiv) and EDCI'HCl (133 mg, 0.69 mmol, 1.05 equiv) were employed. Flash column chromatography (Si02, 20% EtOAc/hexanes ) afforded 292 mg (86%) of the amide product. 1H NMR {Z, major isomer, 300 MHz, CDC13) δ 8.03 (d, J = 8.5 Hz, 1H) , 7.83 (d, J = 1.9 Hz, 1H) , 7.72 (dd, J = 8.4, 1.9 Hz, 1H) , 7.41 - 7.19 (m, 5H) , 7.11 (d, J = 7.9 Hz, 2H) , 7.00 (d, J = 8.4 Hz, 2H) , 6.87 '(d, J = 11.4 Hz, 1H) , 6.81 (d, J = 8.5 Hz, 2H) , 6.08 (dt, J = 11.4, 7.5 Hz, 1H) , 4.85 (td, J = 7.4, 3.5 Hz, 1H) , 3.44 - 3.33 (m, 2H) , 2.88 - 2.73 (m, 2H) , 2.38 (ddd, J = 13.2, 9.4, 5.3 Hz, 1H) , 2.22 (dd, J = 14.6, 7.4 Hz, 1H) , 1.61 (s, 9H) . |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping